Question: Choose the correct statement among the following. (A) Enthalpy of formation of a compound always have positive value. (B) Standard enthalpy of formation of
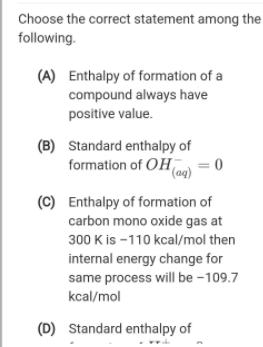
Choose the correct statement among the following. (A) Enthalpy of formation of a compound always have positive value. (B) Standard enthalpy of formation of OHmo = 0 (C) Enthalpy of formation of carbon mono oxide gas at 300 K is -110 kcal/mol then internal energy change for same process will be -109.7 kcal/mol (D) Standard enthalpy of
Step by Step Solution
3.50 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


