Question: Which of the following expression for % ionization of a monobasic weak acid in aqueous solution at appreciable concentration is not correct? K. (A)
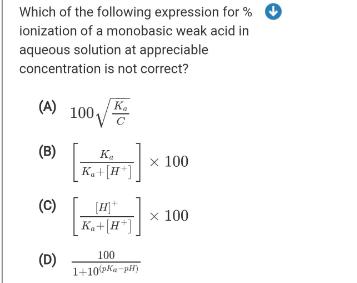
Which of the following expression for % ionization of a monobasic weak acid in aqueous solution at appreciable concentration is not correct? K. (A) 100 (B) K.+[H K. x 100 (C) K,+|H*] x 100 100 (D) 1+10(ka-pil)
Step by Step Solution
There are 3 Steps involved in it
To determine which expression for the ionization of a monobasic weak acid is not correct we need to ... View full answer

Get step-by-step solutions from verified subject matter experts


