Question: answer for 1 please Use the data in the table below to determine the rate law for a reaction with reactants A& & B. The
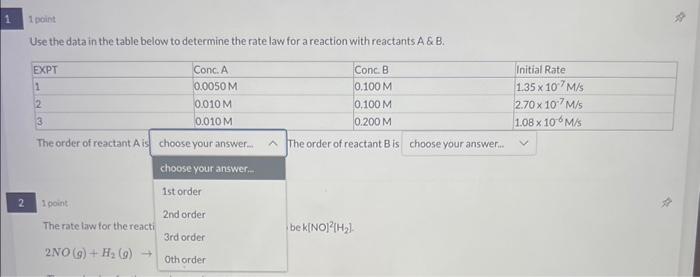
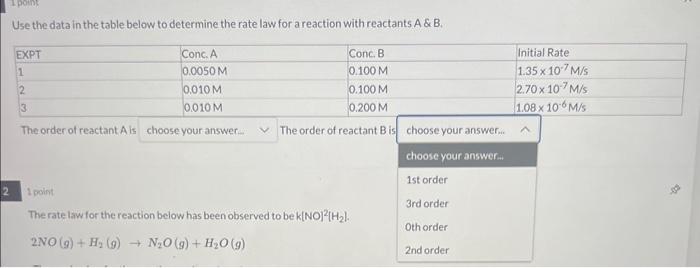
Use the data in the table below to determine the rate law for a reaction with reactants A& \& B. The order of reactant A is The order of reactant B is 1 point The rate law tor the react bek[NO 2[H2] Use the data in the table below to determine the rate law for a reaction with reactants A \& B. The order of reactant A is The order of reactant B is 1 point The rate law for the reaction below has been observed to be k(NO) 2[H2]. 2NO(g)+H2(g)N2O(g)+H2O(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


