Question: answer only please Which two compounds react to form an ester? a. a carboxylic acid and an alcohol b. an alcohol and an aldehyde .
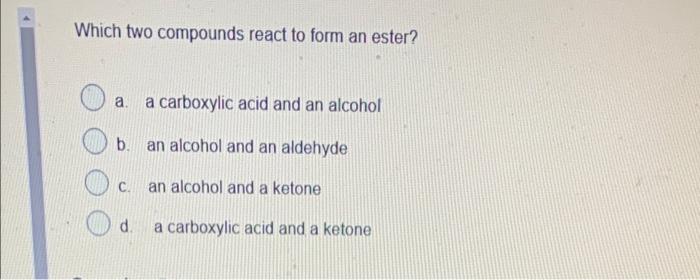
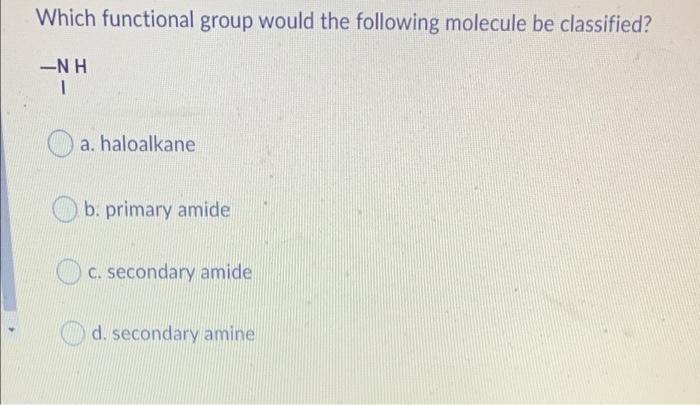
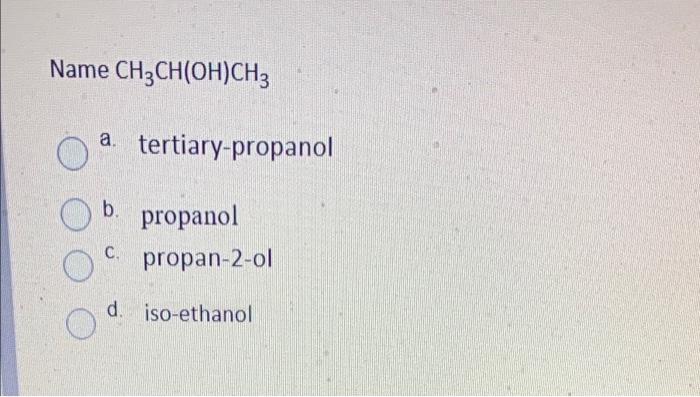
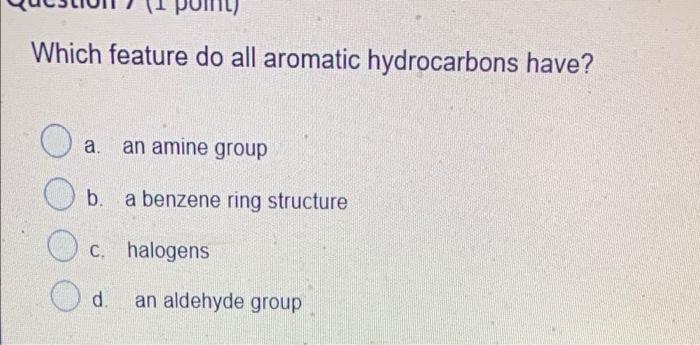



Which two compounds react to form an ester? a. a carboxylic acid and an alcohol b. an alcohol and an aldehyde . an alcohol and a ketone d a carboxylic acid and a ketone Which functional group would the following molecule be classified? -NH 1 a. haloalkane b. primary amide c. secondary amide d. secondary amine Name CH3 CH(OH)CH3 tertiary-propanol b. propanol propan-2-ol C. d. iso-ethanol Which feature do all aromatic hydrocarbons have? a. an amine group b. a benzene ring structure C. halogens d. an aldehyde group Question 8 (1 point) Which compound is formed by the oxidation of a tertiary alcohol? O a a. Aldehyde b. Carboxylic acid c. Ketone d. None Which compound is more soluble in water with same numbers of carbons a. alcohol b. ketone c. alkane d. amine Which is the name of the functional group shown below? R-O-R a. Alcohol . b. Ether c. Aldehyde d. Ester
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


