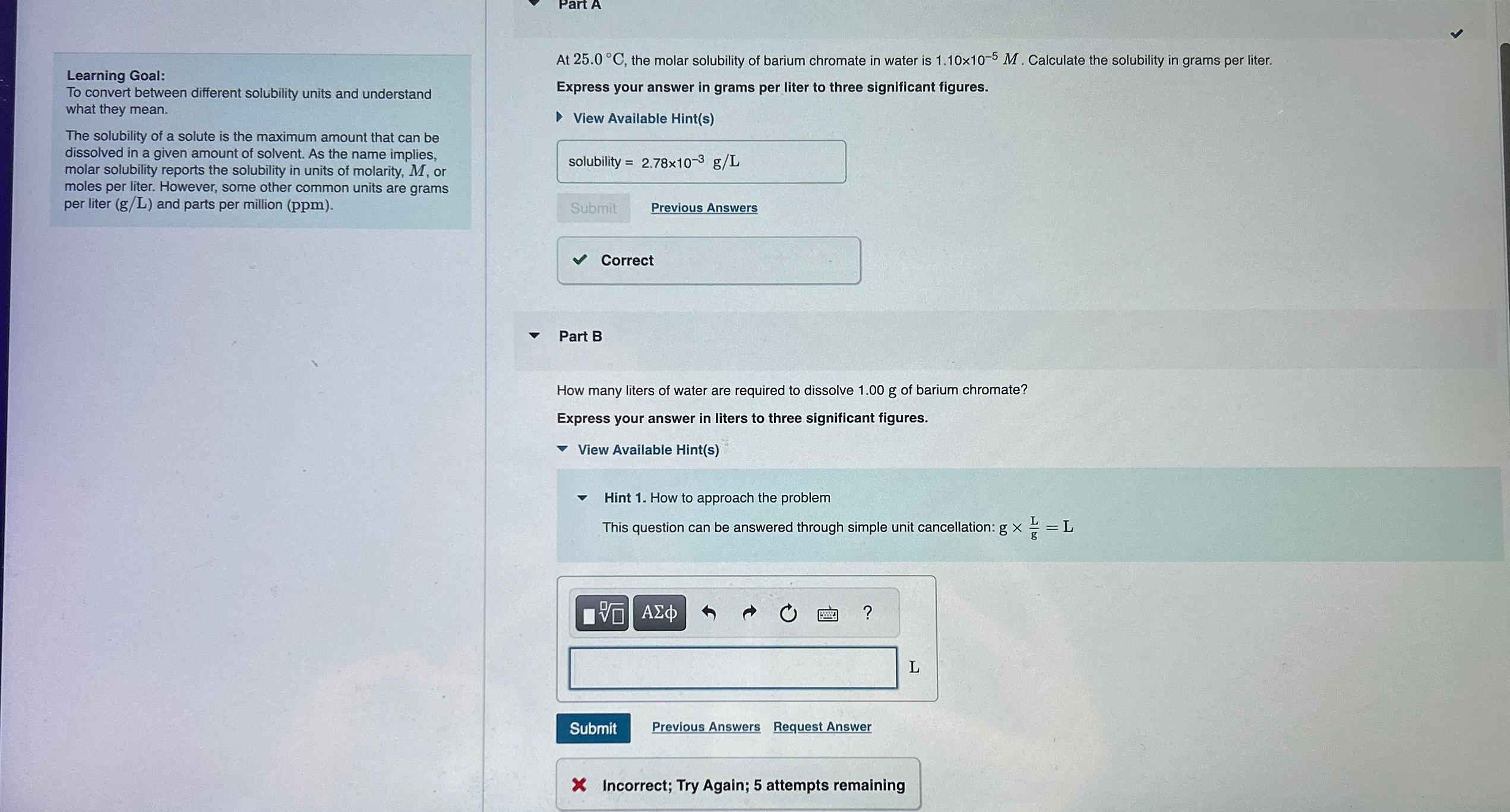
Question: Answer Part B please. Learning Goal: To convert between different solubility units and understand what they mean. The solubility of a solute is the maximum
Answer Part B please.
Learning Goal:
To convert between different solubility units and understand what they mean.
The solubility of a solute is the maximum amount that can be dissolved in a given amount of solvent. As the name implies, molar solubility reports the solubility in units of molarity, or moles per liter. However, some other common units are grams per liter and parts per million
At the molar solubility of barium chromate in water is Calculate the solubility in grams per liter.
Express your answer in grams per liter to three significant figures.
View Available Hints
Submit
Previous Answers
Part B
How many liters of water are required to dissolve of barium chromate?
Express your answer in liters to three significant figures.
View Available Hints
Hint How to approach the problem
This question can be answered through simple unit cancellation:
Request Answer
Incorrect; Try Again; attempts remaining
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


