Question: answer please! Suppose that A and B react to form C according to the equation below. A+2BC What are the equilibrium concentrations of A, B
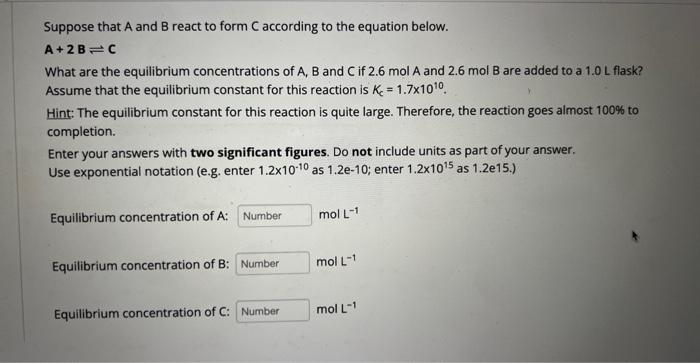
Suppose that A and B react to form C according to the equation below. A+2BC What are the equilibrium concentrations of A, B and C if 2.6molA and 2.6molB are added to a 1.0L flask? Assume that the equilibrium constant for this reaction is Kc=1.71010. Hint: The equilibrium constant for this reaction is quite large. Therefore, the reaction goes almost 100% to completion. Enter your answers with two significant figures. Do not include units as part of your answer. Use exponential notation (e.g. enter 1.21010 as 1.2e10; enter 1.21015 as 1.2e15.) Equilibrium concentration of A : molL1 Equilibrium concentration of B : molL1 Equilibrium concentration of C : molL1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


