Question: Answer question 2 ASSP please Part 3 a n-pentane 0.000677 1/K butanone 0.000422 1/K 1-butanol 0.0008985 1/K butanal 0.0009310 1/K o -8 n-pentane 5.41 =


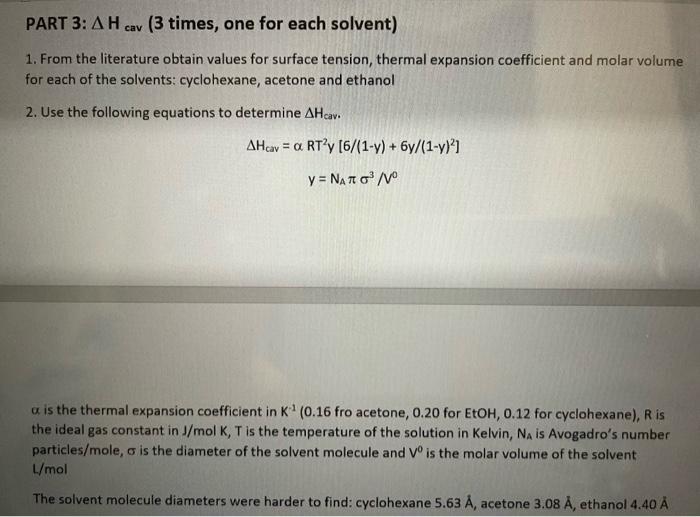
Part 3 a n-pentane 0.000677 1/K butanone 0.000422 1/K 1-butanol 0.0008985 1/K butanal 0.0009310 1/K o -8 n-pentane 5.41 = 5.41 x 10 cm butanone 5.25 = 5.25 x 10 cm -8 1-butanol 4.30 = 4.30 x 10 cm -8 butanal 4.71 = 4.71 x 10 cm -8 PART 3: AH cav (3 times, one for each solvent) 1. From the literature obtain values for surface tension, thermal expansion coefficient and molar volume for each of the solvents: cyclohexane, acetone and ethanol 2. Use the following equations to determine AHeav. AHav = a RT?y [6/(1-7) + 6y/(1-y)? y = Nano/V a is the thermal expansion coefficient in K (0.16 fro acetone, 0.20 for EtOH, 0.12 for cyclohexane), Ris the ideal gas constant in J/mol K, T is the temperature of the solution in Kelvin, NA is Avogadro's number particles/mole, is the diameter of the solvent molecule and Vis the molar volume of the solvent L/mol The solvent molecule diameters were harder to find: cyclohexane 5.63 , acetone 3.08 A, ethanol 4.40
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


