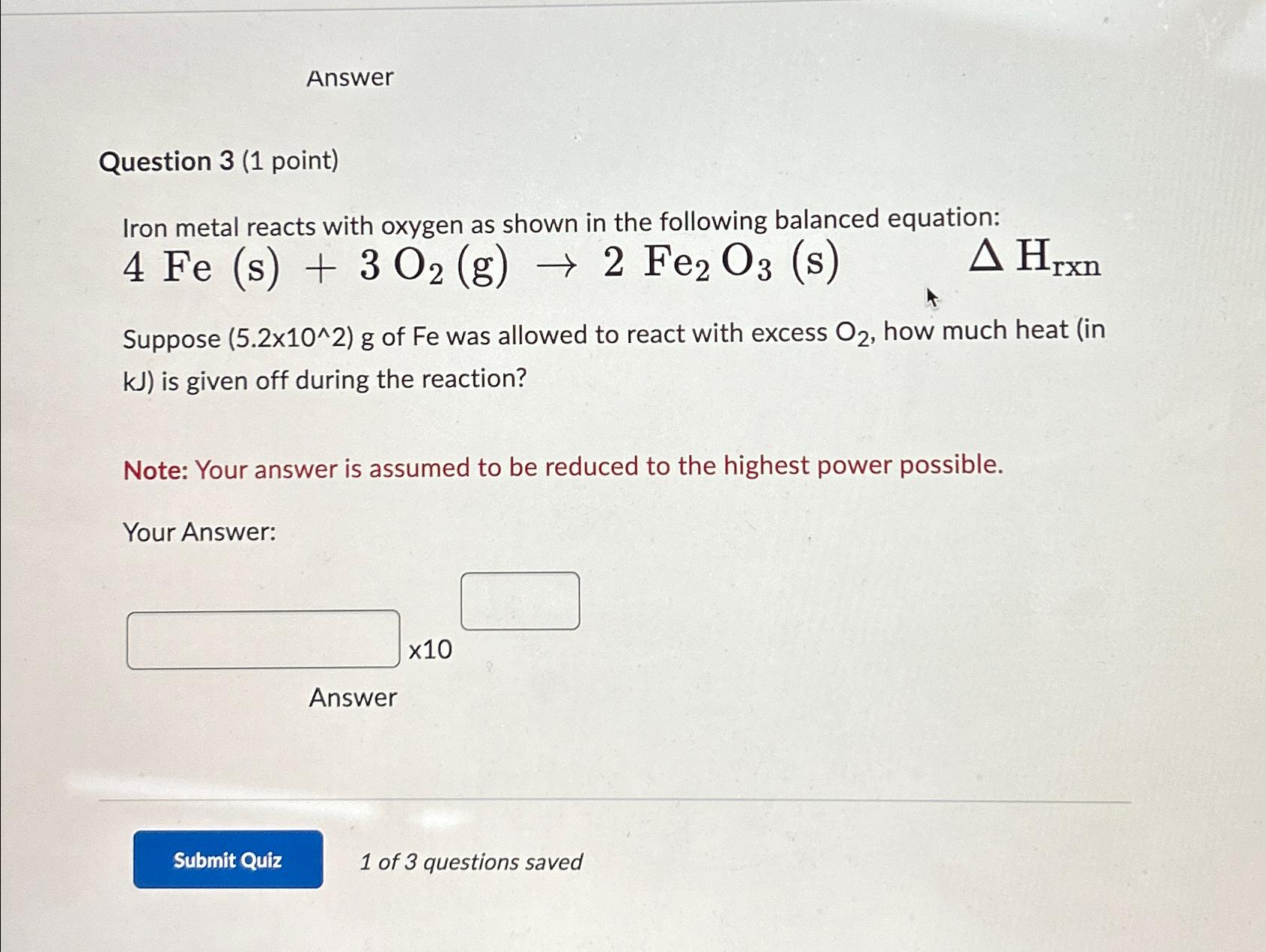
Question: Answer Question 3 ( 1 point ) Iron metal reacts with oxygen as shown in the following balanced equation: 4 F e ( s )
Answer
Question point
Iron metal reacts with oxygen as shown in the following balanced equation:
kJ
Suppose of Fe was allowed to react with excess how much heat in is given off during the reaction?
Note: Your answer is assumed to be reduced to the highest power possible.
Your Answer:
Answer
of questions saved
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


