Question: Answer Questions 2-3 2. Using the formula for the ideal gas law and the value for the gas law constant of 0.08206 L'atm-k-1.mol-1 calculate the

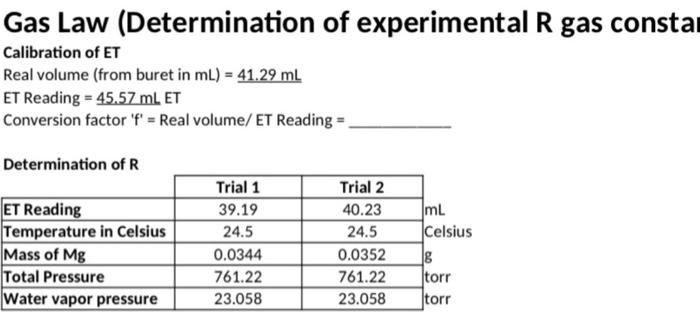
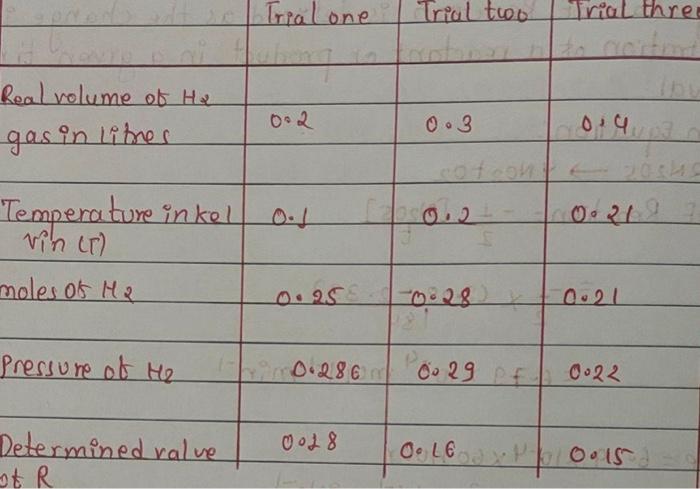
2. Using the formula for the ideal gas law and the value for the gas law constant of 0.08206 L'atm-k-1.mol-1 calculate the volume of one mole of any gas at STP. (STP means 273.15 Kand one atmosphere pressure). 3. Using the formula for the ideal gas law and the value for the gas law constant of 0.08206 L'atm K-1.mol-1, what is the volume of 5.05 grams of dry hydrogen at 25.0*C and 758 torr? Gas Law (Determination of experimental R gas consta Calibration of ET Real volume (from buret in ml) = 41.29 mL ET Reading = 45.57 mL ET Conversion factor 'f' = Real volume/ ET Reading = Determination of R ET Reading Temperature in Celsius Mass of Mg Total Pressure Water vapor pressure Trial 1 39.19 24.5 0.0344 761.22 23.058 Trial 2 40.23 24.5 0.0352 761.22 23.058 mL Celsius g torr torr Trial one Trial two Trial three Real volume of H2 0.2 0.3 gas in liter 0:4 0.2 021.9 Temperature inkel vin it) moles os ha 0.25 028 0.21 Pressure of H2 0.286 00 29 of 0.22 Determined value 0018 O. LE Oos StR
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


