Question: pls answer will give thumbs up!! Part A When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reaction CaCO3(4)

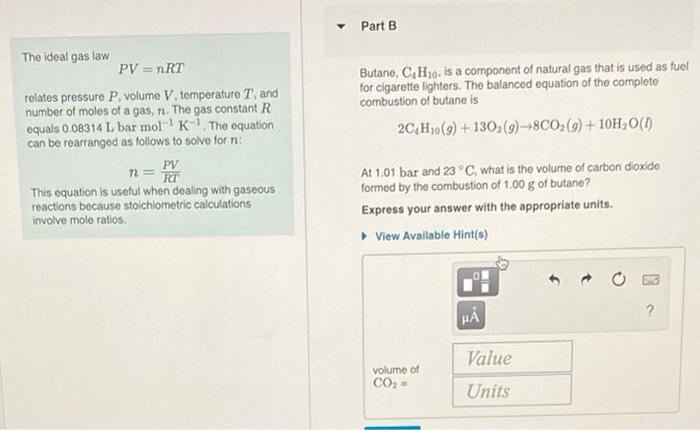
Part A When heated, calcium carbonate decomposes to yield calcium oxide and carbon dioxide gas via the reaction CaCO3(4) Ca (8) + CO3(8) The ideal gas law PV = nRT relates pressure P. volume V temperature T, and number of moles of a gas. n. The gas constant R equals 0.08314 L bar mol K-The equation can be rearranged as follows to solve for na PV n- RT This equation is useful when dealing with gaseous reactions because stoichiometric calculations involve mole ratios What is the mass of calcium carbonate needed to produce 810 L of carbon dioxide at 1 bar and 273 K? Express your answer with the appropriate units. View Available Hint(s) H Value mass of CaCO, Units Part B The ideal gas law PV = nRT relates pressure P. volume V temperature T, and number of moles of a gas, n. The gas constant R equals 0.08314 L bar mol' K The equation can be rearranged as follows to solve for ni: PV n = RT This equation is useful when dealing with gaseous reactions because stoichiometric calculations involve mole ratios Butane, CH30, is a component of natural gas that is used as fuel for cigarette lighters. The balanced equation of the complete combustion of butane is 2C,H10(9) + 130;(9)+8C02(9) +10H20(1) At 1.01 bar and 23 C, what is the volume of carbon dioxide formed by the combustion of 1,00 g of butane? Express your answer with the appropriate units. View Available Hint(s) H ? Value volume of CO2- Units
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


