Question: Answer quickly for thumbs up (Answer is 16.89x10^5 KJ) (b) Calculate the heat required to bring 300kg of solid sodium from 0C to a vapour
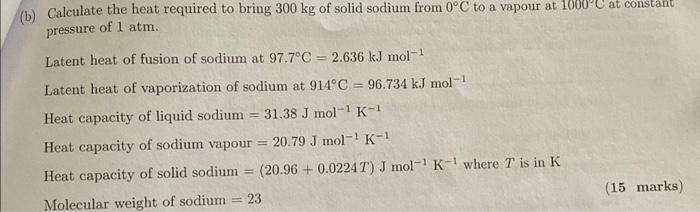
(b) Calculate the heat required to bring 300kg of solid sodium from 0C to a vapour at 1000C at constaim pressure of 1atm. Latent heat of fusion of sodium at 97.7C=2.636kJmol1 Latent heat of vaporization of sodium at 914C=96.734kJmol1 Heat capacity of liquid sodium =31.38Jmol1K1 Heat capacity of sodium vapour =20.79Jmol1K1 Heat capacity of solid sodium =(20.96+0.0224T)Jmol1K1 where T is in K Molecular weight of sodium =23 (15 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


