Question: Answer quickly for thumbs up (Answer is T= 1481.3 deg celcius) Calculate the theoretical flame temperature for CO burnt at 110kPa absolute pressure with 100%
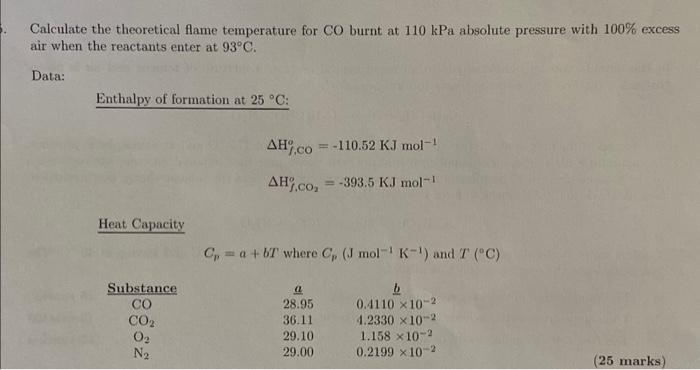
Calculate the theoretical flame temperature for CO burnt at 110kPa absolute pressure with 100% excess air when the reactants enter at 93C. Data: Enthalpy of formation at 25C : H%,COo=110.52KJmol1H,,CO2o=393.5KJmol1 Heat Capacity Cp=a+bTwhereCp(Jmol1K1)andT(C) Calculate the theoretical flame temperature for CO burnt at 110kPa absolute pressure with 100% excess air when the reactants enter at 93C. Data: Enthalpy of formation at 25C : H%,COo=110.52KJmol1H,,CO2o=393.5KJmol1 Heat Capacity Cp=a+bTwhereCp(Jmol1K1)andT(C)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


