Question: Answer Quickly for thumbs up. . . . . APPARATUS/REAGENTS Orange juice samples (Orchard & 6 250mL. conical flasks Kool Kids) 1 ImL micro-pipettor 0.0005M

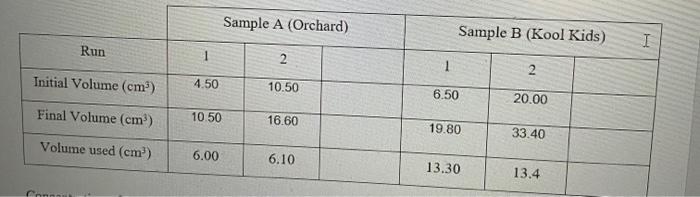
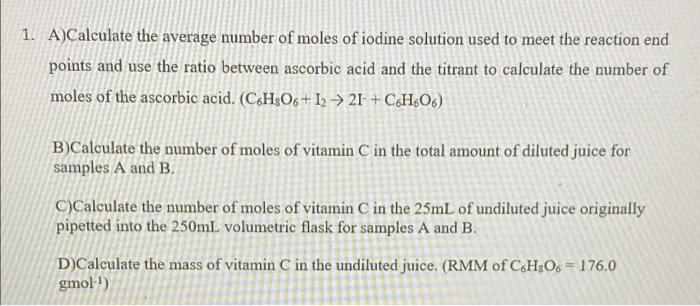
. . . . APPARATUS/REAGENTS Orange juice samples (Orchard & 6 250mL. conical flasks Kool Kids) 1 ImL micro-pipettor 0.0005M iodinc solution 2 25mL pipettes (potassium iodide + iodine) 6 150ml beakers 0.5% starch indicator solution 1 plastic dropper Distilled water 2 250mL volumetric flasks 2 50ml. burettes and retort stands 2 Glass funnels . . . . PROCEDURE 1. Fill the burette with the iodine solution provided just above the OmL mark. Allow some of the iodine solution to flow through the burette tip in order to remove any air bubbles present. Record the initial volume in the burette 2. Label one beaker A and another B. Pour approximately 40mL of Orchard juice into beaker A. 3. Pipette 25ml. of sample A (Orchard) into a labelled 250mL volumetric flask and make up to the mark with distilled water. Mix by inversion until a homogeneous solution is formed. 4. Pour some sample A from the volumetric flask into a beaker and pipette 25mL of the diluted sample into a conical flask. Cover this beaker with parafilm when not in use as ascorbic acid is susceptible to oxidation by atmosphetic oxygen over time. 5. Measure ImL of starch indicator solution using the Iml. pipette and add to the conical flask. Swirl to combine contents 6. Perform the titration until the first appearance of a permanent blue-black colour. Record the final volume in the burette. 7. Perform additional titrations with sample A until you have at least 2 titre values that are +0.10ml. apart. Record all readings 8. Repeat the procedure with sample B (Kool Kids) Sample A (Orchard) Sample B (Kool Kids) I Run 1 2. 1 2 Initial Volume (cm) 4.50 10.50 6.50 20.00 Final Volume (cm) 10.50 16.60 19.80 33.40 Volume used (cm) 6.00 6.10 13.30 13.4 Connau 1. A)Calculate the average number of moles of iodine solution used to meet the reaction end points and use the ratio between ascorbic acid and the titrant to calculate the number of moles of the ascorbic acid. (C6H3O6 + 12 21+ CH.06) B)Calculate the number of moles of vitamin C in the total amount of diluted juice for samples A and B. C)Calculate the number of moles of vitamin C in the 25mL of undiluted juice originally pipetted into the 250mL volumetric flask for samples A and B. D)Calculate the mass of vitamin C in the undiluted juice. (RMM of C6H506 = 176.0 gmol-)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


