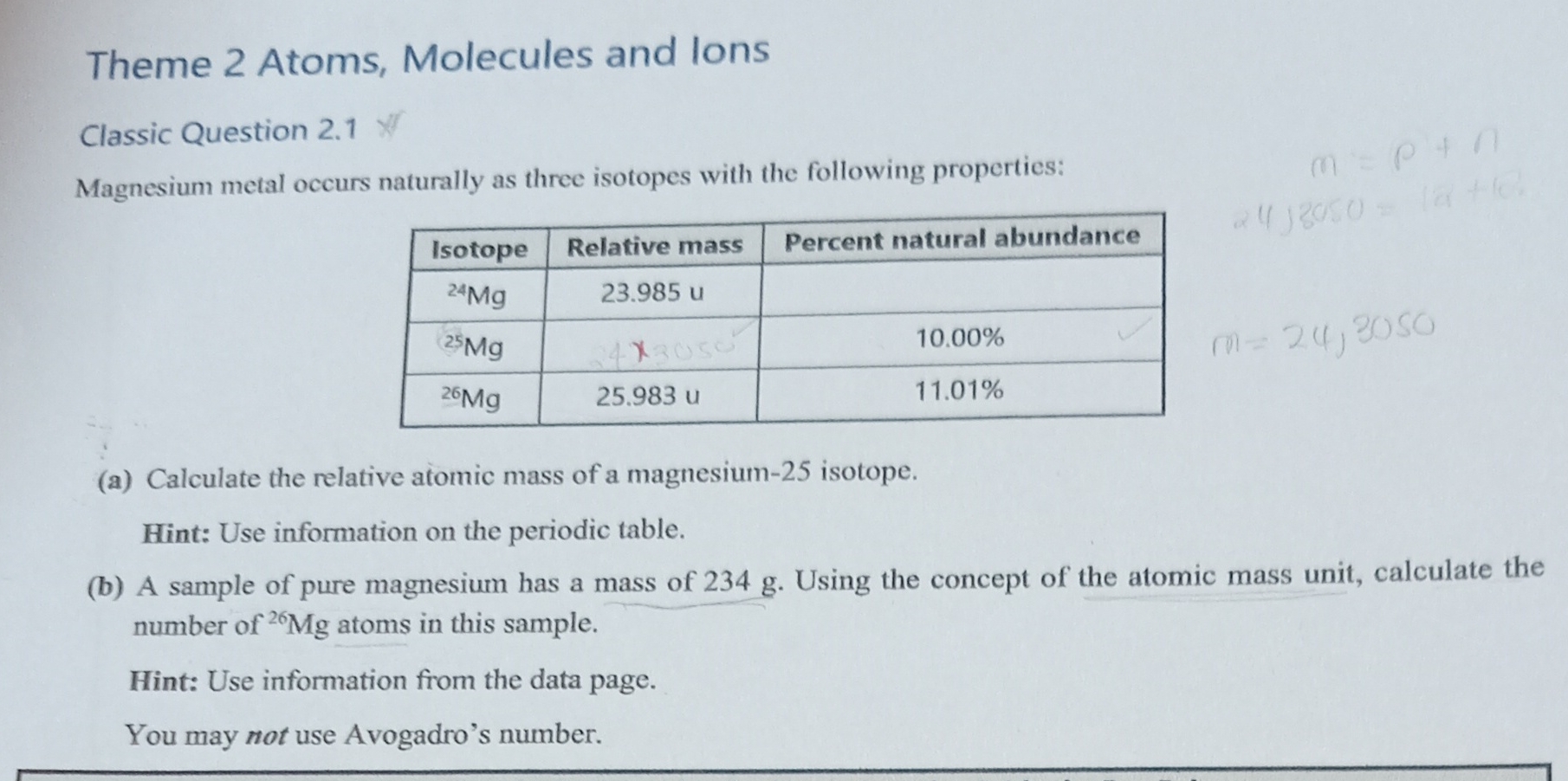
Question: Answer step by step Theme 2 Atoms, Molecules and lons Classic Question 2.1 * Magnesium metal occurs naturally as three isotopes with the following properties:
Answer step by step
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


