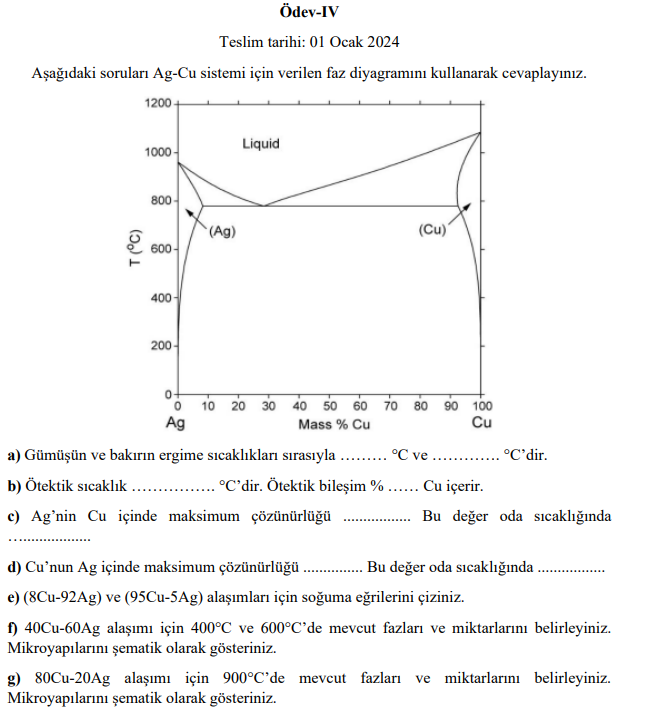
Question: Answer the following questions using the phase diagram given for the Ag - Cu system. a ) Melting temperatures of silver and copper, respectively
Answer the following questions using the phase diagram given for the AgCu system.
a Melting temperatures of silver and copper, respectively
deg C and
deg C
b Eutectic temperature
deg C Eutectic composition
Contains Cu
c Maximum solubility of Ag in Cu
This value is at room temperature
d Maximum solubility of Cu in Ag
This value is at room temperature
eDraw the cooling curves for CuAg and CuAg alloys.
f Determine the phases and their quantities present at deg C and deg C for the CuAg alloy.
Show their microstructures schematically.
g Determine the phases and their quantities present at deg C for the CuAg alloy.
Show their microstructures schematically.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


