Question: Answer these questions. Physical constants which may be necessary to answer the problems on this page can be found within the hint tabs. Don't forget
Answer these questions.
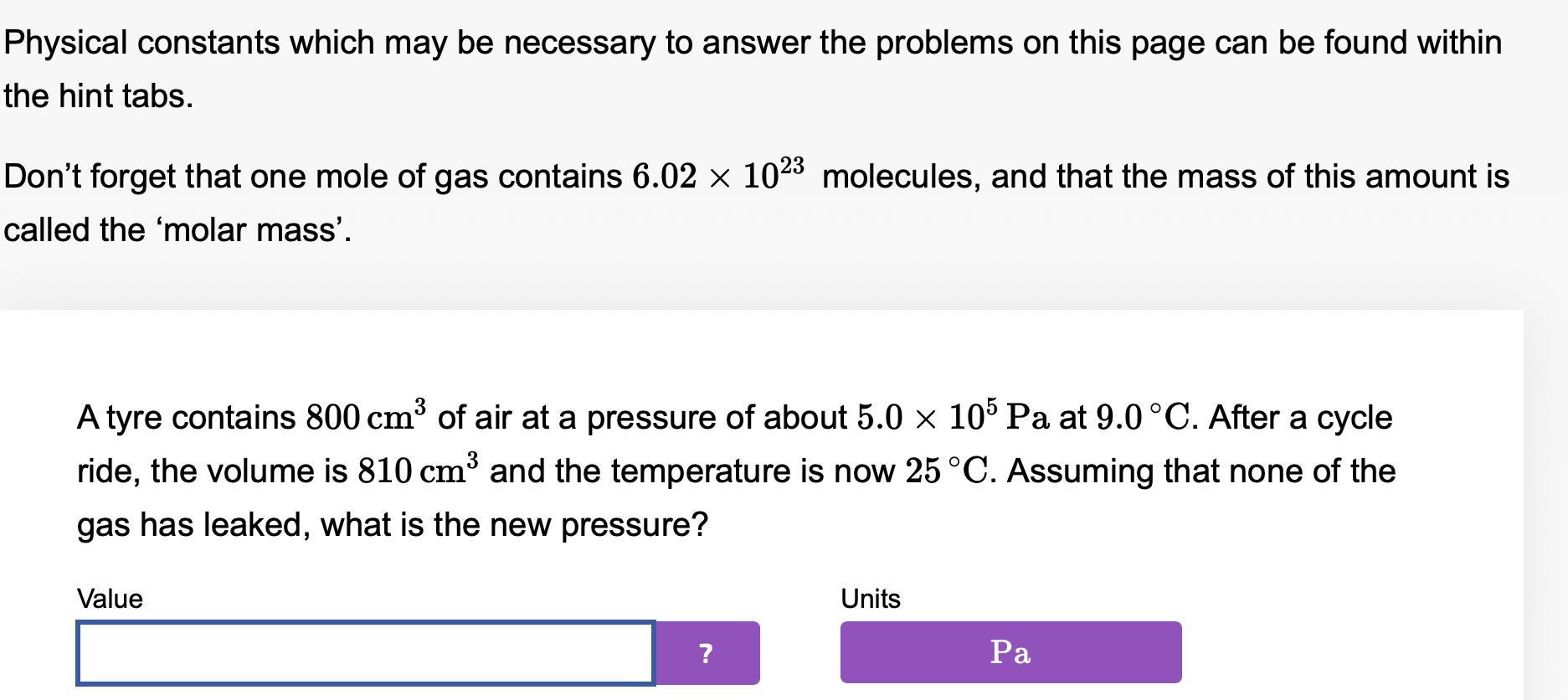
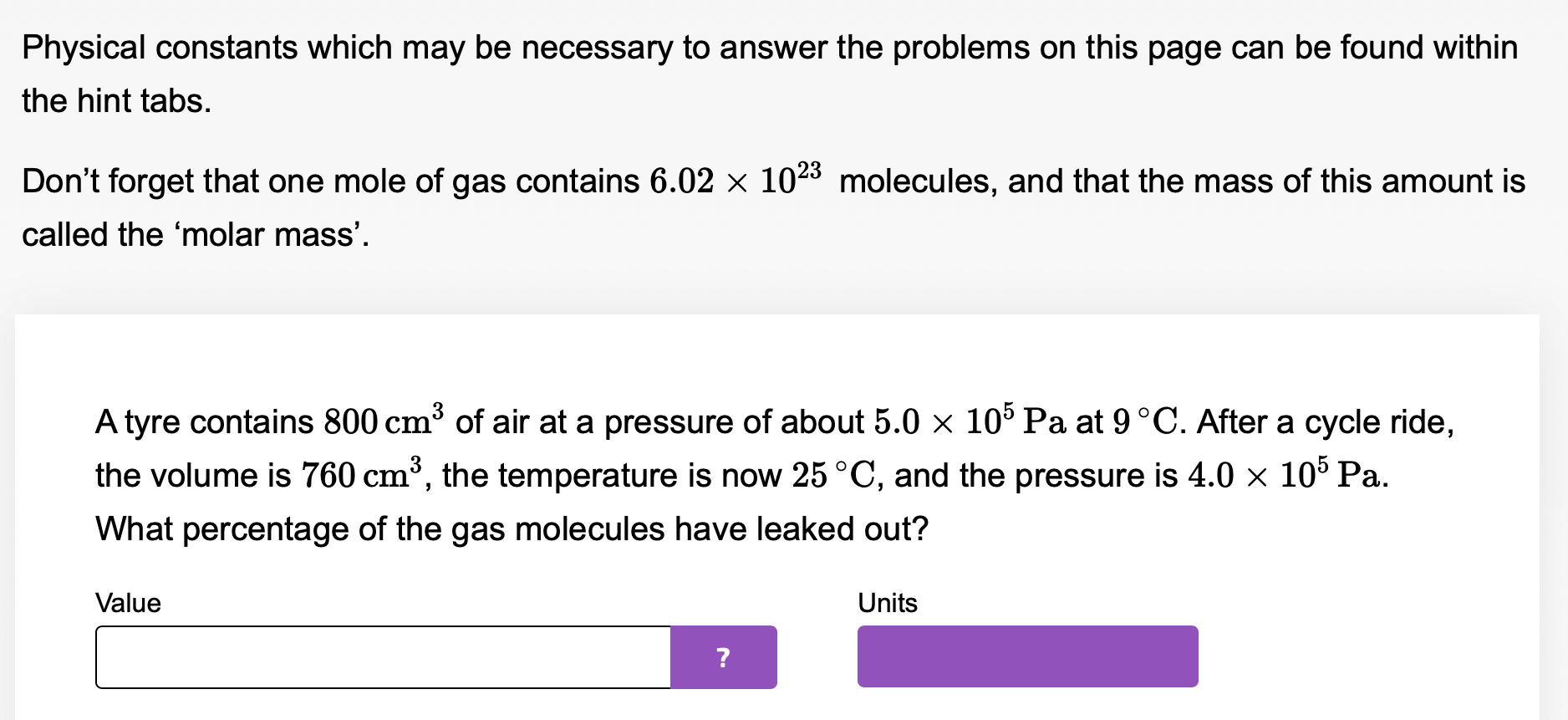
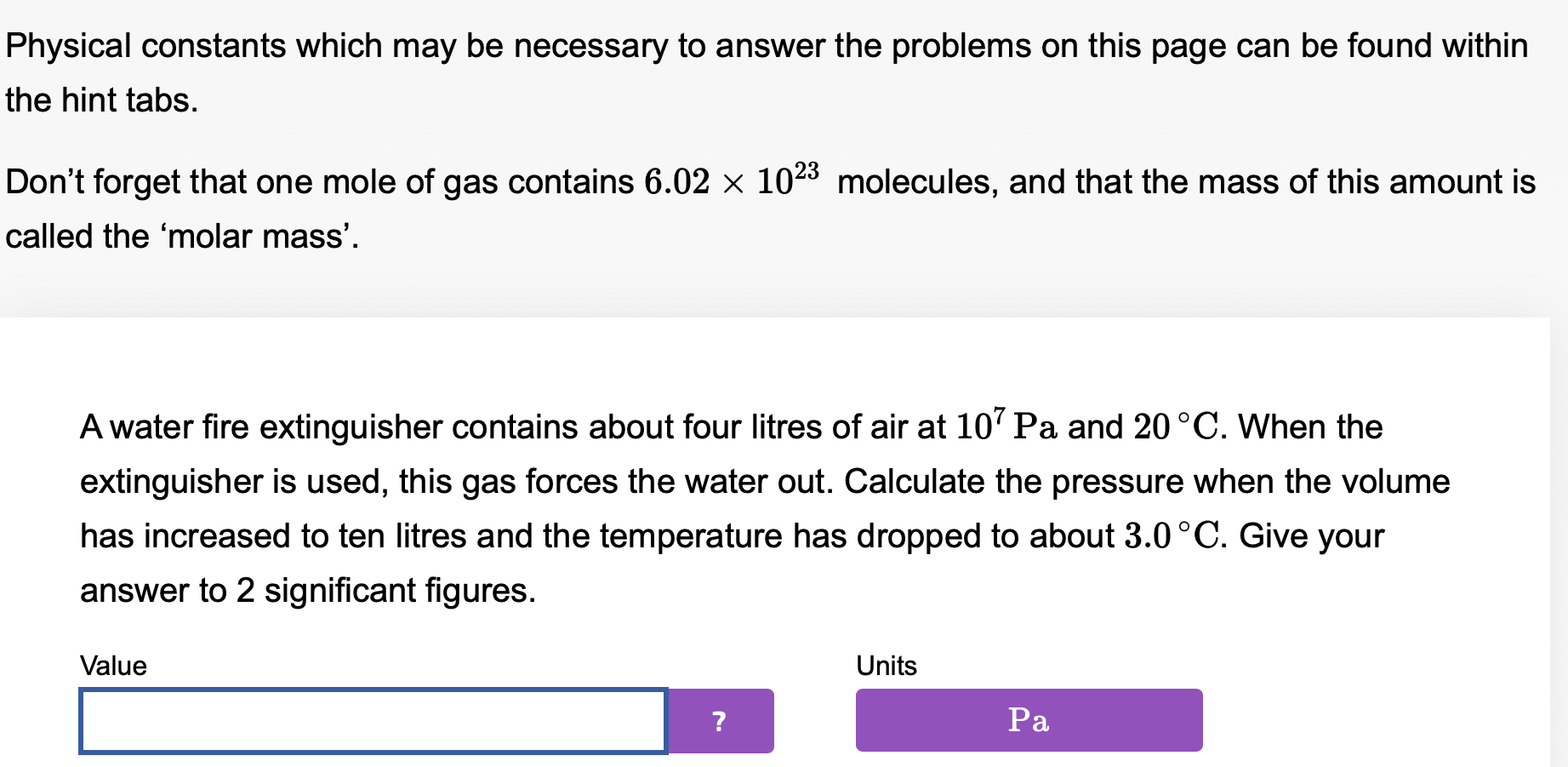
Physical constants which may be necessary to answer the problems on this page can be found within the hint tabs. Don't forget that one mole of gas contains 6.02 x 1023 molecules, and that the mass of this amount is called the 'molar mass'. Atyre contains 800 cm3 of air at a pressure of about 5.0 x 105 Pa at 9.0 C. After a cycle ride, the volume is 810 cm3 and the temperature is now 25 OC. Assuming that none of the gas has leaked, what is the new pressure? Value Units Physical constants which may be necessary to answer the problems on this page can be found within the hint tabs. Don't forget that one mole of gas contains 6.02 x 1023 molecules, and that the mass of this amount is called the 'molar mass'. Atyre contains 800 cm3 of air at a pressure of about 5.0 x 105 Pa at 9 C. After a cycle ride, the volume is 760 cm3, the temperature is now 25 OC, and the pressure is 4.0 x 105 Pa. What percentage of the gas molecules have leaked out? Value Units . Physical constants which may be necessary to answer the problems on this page can be found within the hint tabs. Don't forget that one mole of gas contains 6.02 X 1023 molecules, and that the mass of this amount is called the 'molar mass'. A water re extinguisher contains about four litres of air at 107 Pa and 20 C. When the extinguisher is used, this gas forces the water out. Calculate the pressure when the volume has increased to ten litres and the temperature has dropped to about 3.0 C. Give your answer to 2 signicant gures. Value Units \
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


