Question: As discussed in the video 6-3, epoxides can react by both an SN1 or an SN2 mechanism, depending on the type of nucleophile, the solvent,
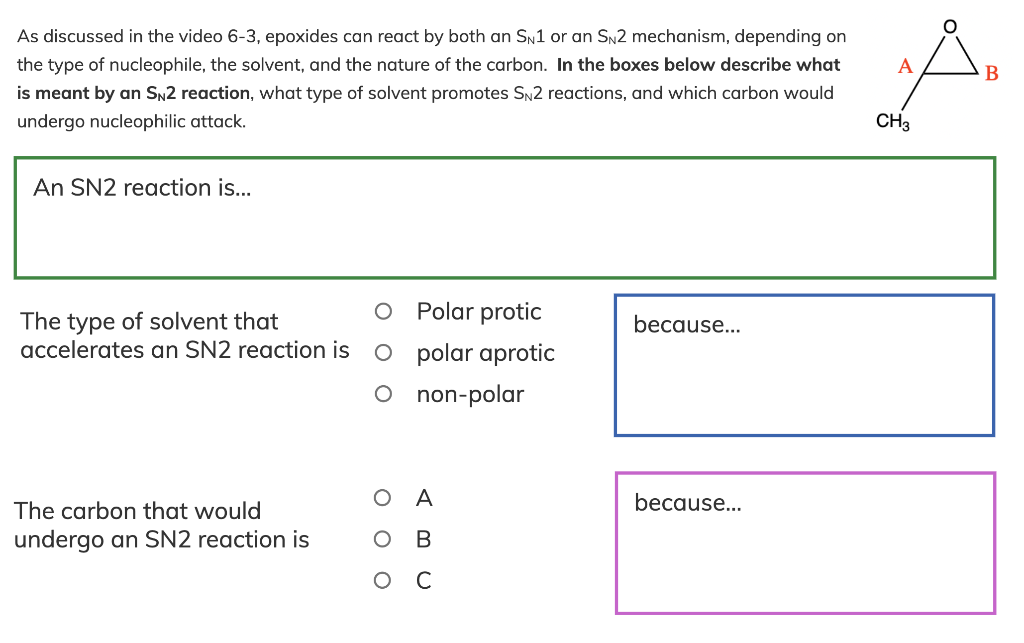
As discussed in the video 6-3, epoxides can react by both an SN1 or an SN2 mechanism, depending on the type of nucleophile, the solvent, and the nature of the carbon. In the boxes below describe what is meant by an SN2 reaction, what type of solvent promotes SN2 reactions, and which carbon would undergo nucleophilic attack. An SN2 reaction is... The type of solvent that Polar protic accelerates an SN2 reaction is polar aprotic non-polar The carbon that would A undergo an SN2 reaction is B C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


