Question: As shown in the figure below, you have a system including a Beryllium cup with mass Mcup = 0.359 kg. The cup contains water
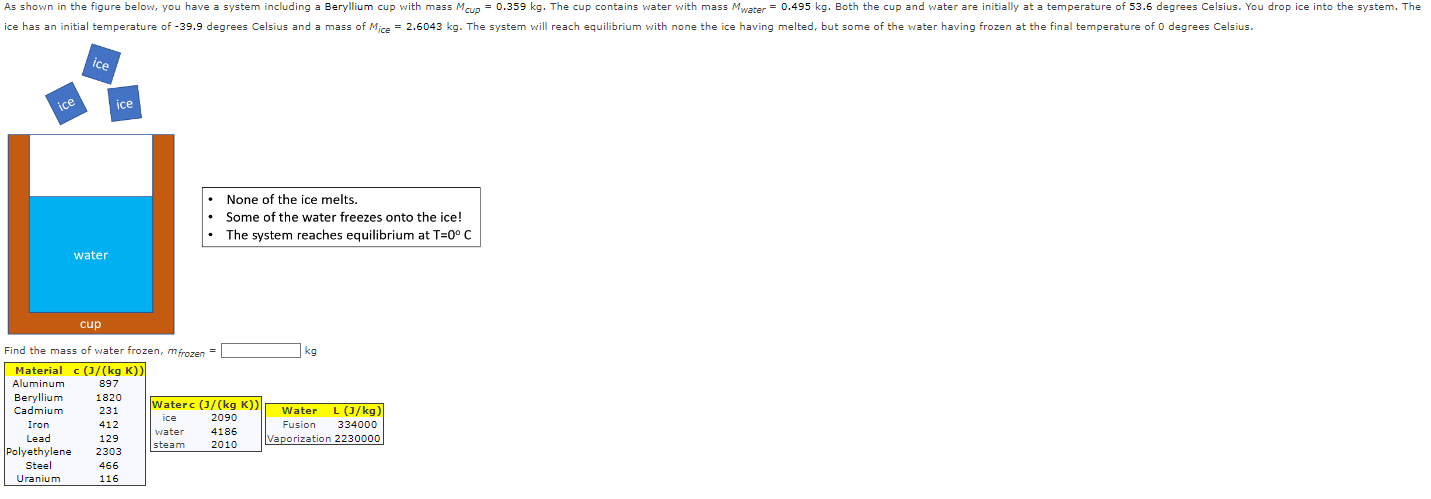
As shown in the figure below, you have a system including a Beryllium cup with mass Mcup = 0.359 kg. The cup contains water with mass Mwater = 0.495 kg. Both the cup and water are initially at a temperature of 53.6 degrees Celsius. You drop ice into the system. The ice has an initial temperature of -39.9 degrees Celsius and a mass of Mice = 2.6043 kg. The system will reach equilibrium with none the ice having melted, but some of the water having frozen at the final temperature of 0 degrees Celsius. ice ice ice water None of the ice melts. Some of the water freezes onto the ice! The system reaches equilibrium at T=0 C cup Find the mass of water frozen, mfrozen Material c (J/(kg K)) kg Aluminum 897 Beryllium 1820 Cadmium 231 Waterc (J/(kg K)) ice 2090 Iron 412 water 4186 Lead 129 steam Water L (J/kg) Fusion 334000 Vaporization 2230000 2010 Polyethylene 2303 Steel 466 Uranium 116
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


