Question: Assuming the van't Hoff factors in the table below, calculate the mass of solute required to make each aqueous solution: Table. Van't Hoff Factors
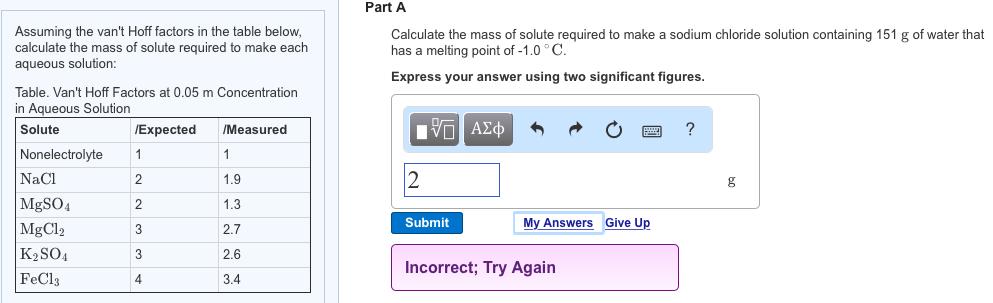
Assuming the van't Hoff factors in the table below, calculate the mass of solute required to make each aqueous solution: Table. Van't Hoff Factors at 0.05 m Concentration in Aqueous Solution Solute Nonelectrolyte NaCl MgSO4 MgCl2 KSO4 FeCl3 /Expected 1 2 2 3 3 4 /Measured 1 1.9 1.3 2.7 2.6 3.4 Part A Calculate the mass of solute required to make a sodium chloride solution containing 151 g of water that has a melting point of -1.0C. Express your answer using two significant figures. 15 2 Submit My Answers Give Up Incorrect; Try Again ?
Step by Step Solution
3.37 Rating (156 Votes )
There are 3 Steps involved in it
p M M ZxM a x No 80g cm For b... View full answer

Get step-by-step solutions from verified subject matter experts


