Question: Using the van't Hoff factors in the table below, calculate the mass of solute required to make each aqueous solution. Van't Hoff factors at 0.05m
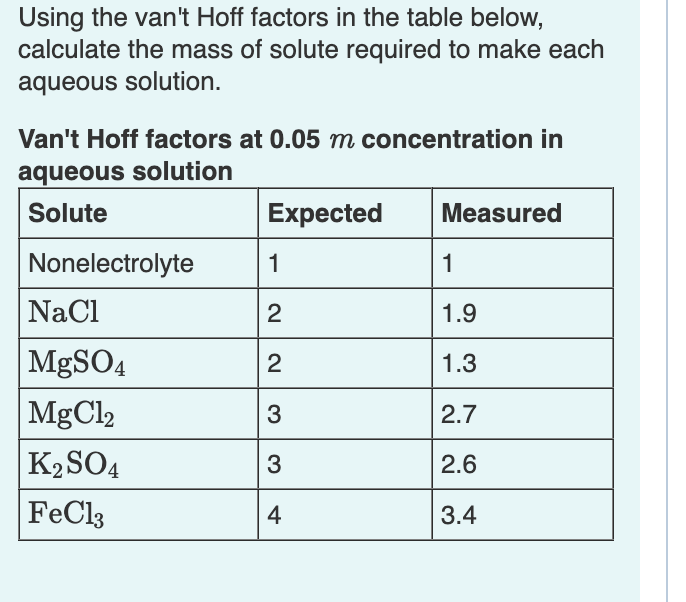
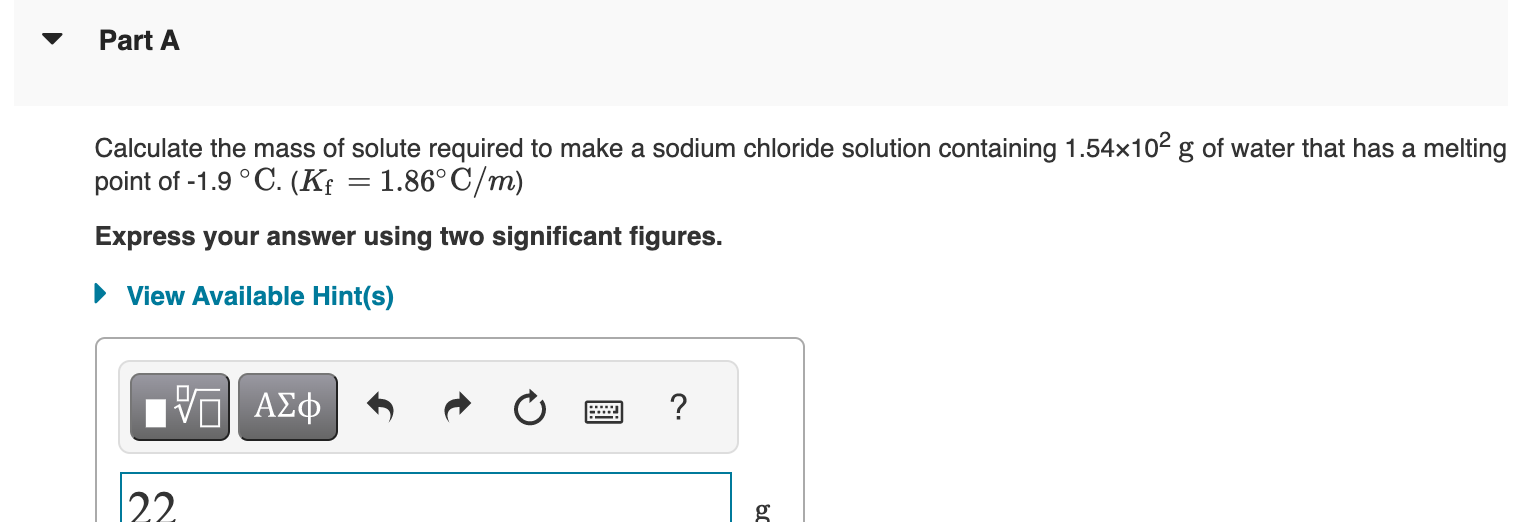
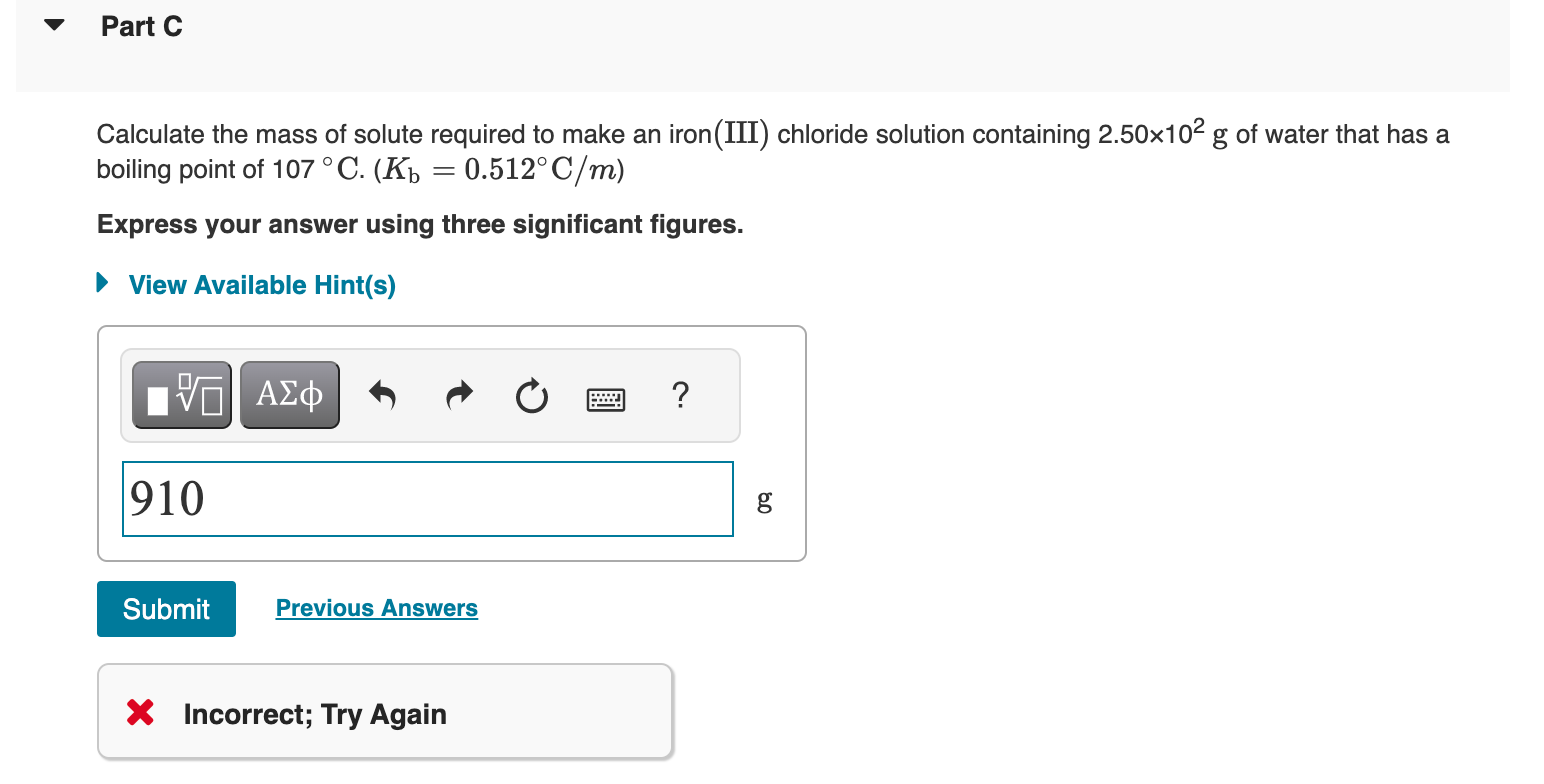
Using the van't Hoff factors in the table below, calculate the mass of solute required to make each aqueous solution. Van't Hoff factors at 0.05m concentration in aqueous solution Calculate the mass of solute required to make a sodium chloride solution containing 1.54102g of water that has a melting point of 1.9C.(Kf=1.86C/m) Express your answer using two significant figures. Calculate the mass of solute required to make an iron(III) chloride solution containing 2.50102g of water that has a boiling point of 107C.(Kb=0.512C/m) Express your answer using three significant figures. X Incorrect; Try Again
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


