Question: Assumptions: The following variables have constant values in this problem: F, V, T1 and T2. The reactors are well mixed. 1. 2. 3. The
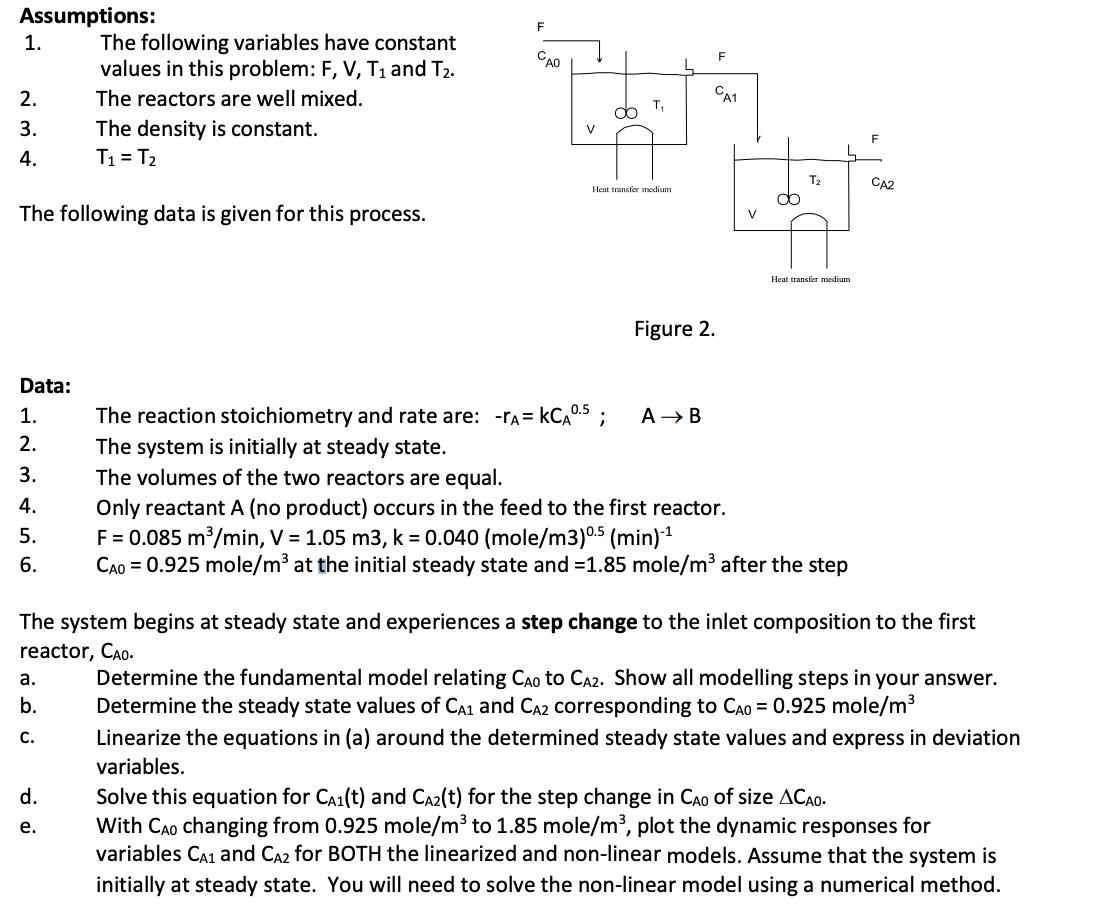
Assumptions: The following variables have constant values in this problem: F, V, T1 and T2. The reactors are well mixed. 1. 2. 3. The density is constant. 4. T = T2 F CAO V T The following data is given for this process. Heat transfer medium Figure 2. CA1 V F T CA2 Heat transfer medium. Data: 1. The reaction stoichiometry and rate are: -r = KCA 0.5; AB 2. The system is initially at steady state. 3. The volumes of the two reactors are equal. 4. Only reactant A (no product) occurs in the feed to the first reactor. 5. F = 0.085 m/min, V = 1.05 m3, k = 0.040 (mole/m3) 0.5 (min)-1 6. CAO 0.925 mole/m at the initial steady state and -1.85 mole/m after the step The system begins at steady state and experiences a step change to the inlet composition to the first reactor, CAO. a. b. C. Determine the fundamental model relating CAD to CA2. Show all modelling steps in your answer. Determine the steady state values of CA1 and CA2 corresponding to CAD = 0.925 mole/m Linearize the equations in (a) around the determined steady state values and express in deviation variables. d. Solve this equation for CA(t) and CA2(t) for the step change in CAO of size ACAO. PP e. With CAO changing from 0.925 mole/m to 1.85 mole/m, plot the dynamic responses for variables CA1 and CA2 for BOTH the linearized and non-linear models. Assume that the system is initially at steady state. You will need to solve the non-linear model using a numerical method.
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


