Question: At 25C a binary system containing components A and B is in a state of liquid-liquid vapor equilibrium. The mixture is not ideal however
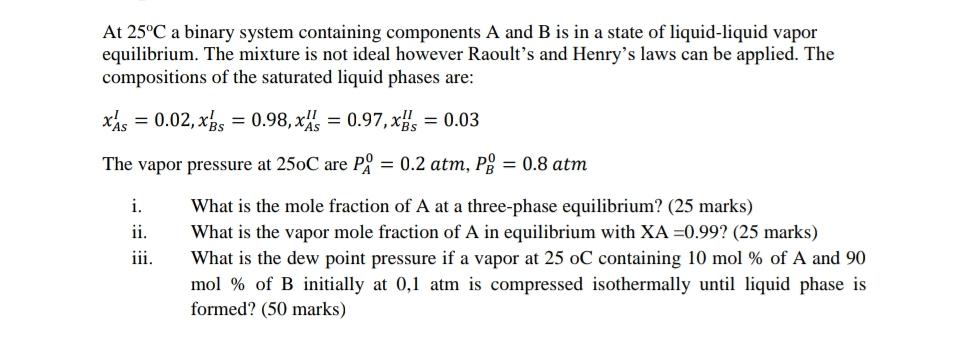
At 25C a binary system containing components A and B is in a state of liquid-liquid vapor equilibrium. The mixture is not ideal however Raoult's and Henry's laws can be applied. The compositions of the saturated liquid phases are: xis = 0.02, xs = 0.98, x = 0.97, x, = 0.03 The vapor pressure at 250C are P = 0.2 atm, P = 0.8 atm i. What is the mole fraction of A at a three-phase equilibrium? (25 marks) What is the vapor mole fraction of A in equilibrium with XA =0.99? (25 marks) What is the dew point pressure if a vapor at 25 oC containing 10 mol % of A and 90 ii. iii. mol % of B initially at 0,1 atm is compressed isothermally until liquid phase is formed? (50 marks)
Step by Step Solution
3.47 Rating (167 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


