Question: At a certain temperature, the equilibrium constant K for the follawing reaction is 320 ,: N2(e)+O2(g)+2NO(g) Use this information to complete the following table
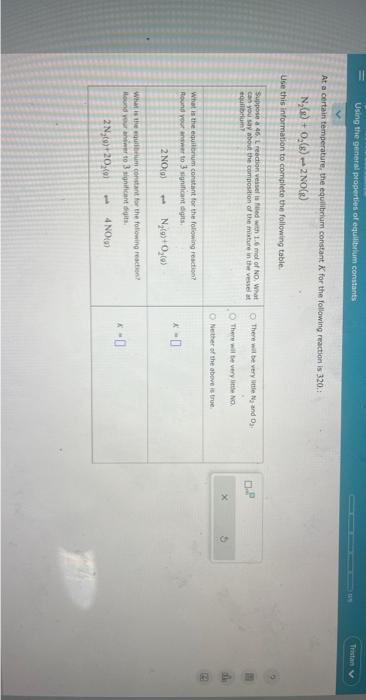
At a certain temperature, the equilibrium constant K for the follawing reaction is 320 ,: N2(e)+O2(g)+2NO(g) Use this information to complete the following table
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


