Question: At a certain temperature, the equilibrium constant K for the following reaction is 8.75 x 1010. C12(g) = 2 HCl(g) H2(g) + Use this information
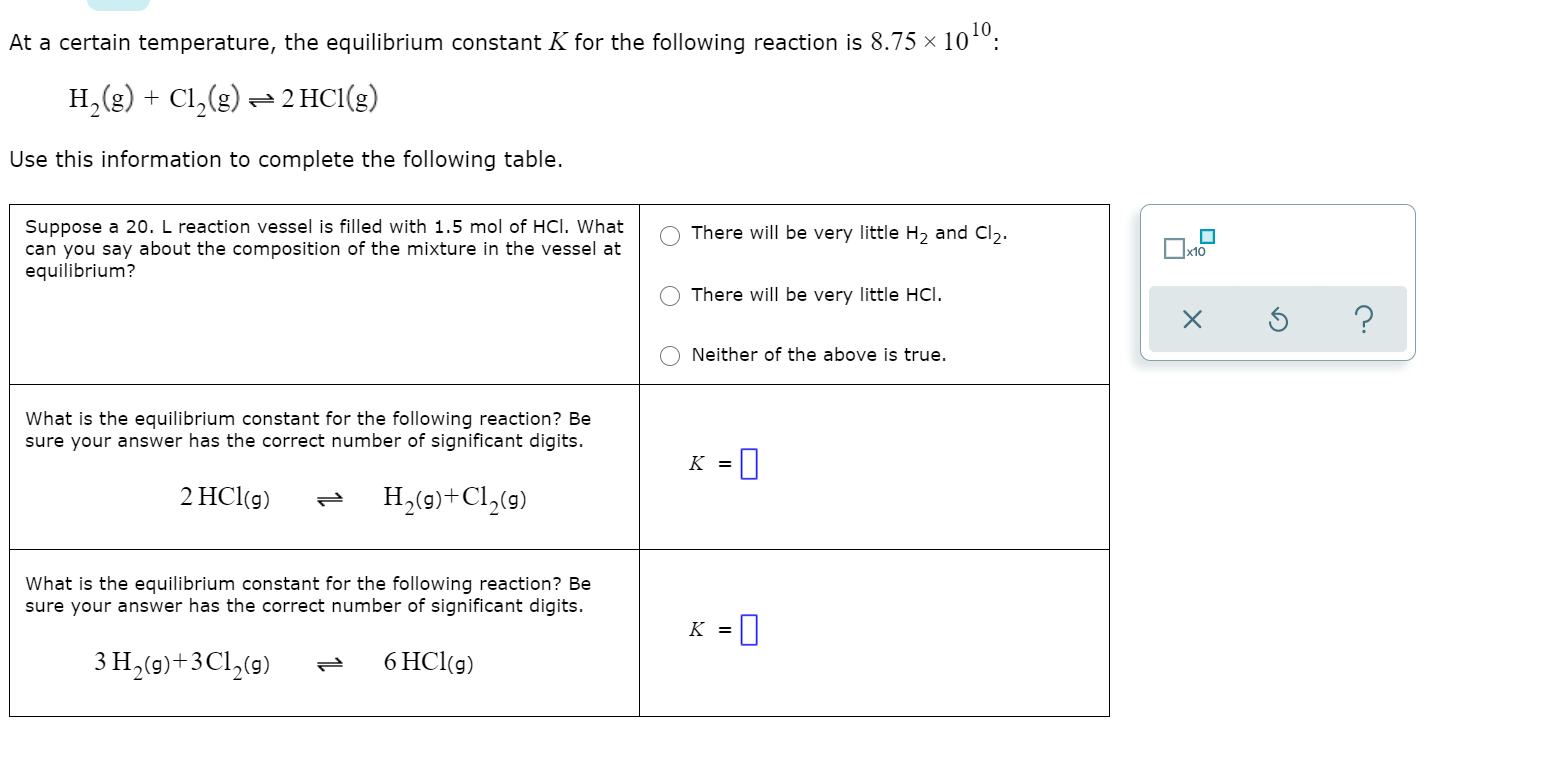
At a certain temperature, the equilibrium constant K for the following reaction is 8.75 x 1010. C12(g) = 2 HCl(g) H2(g) + Use this information to complete the following table. There will be very little Hz and Cl2 Suppose a 20. L reaction vessel is filled with 1.5 mol of HCl. What can you say about the composition of the mixture in the vessel at equilibrium? Txo There will be very little HCl. ? Neither of the above is true. What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K = 2 HCl(g) H (9)+C1 (9) What is the equilibrium constant for the following reaction? Be sure your answer has the correct number of significant digits. K = 1 3 H (9)+3C12(9) 6 HCl(g)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


