During the analysis of an unknown weak base B, a 0.10 m solution of the nitrate salt
Question:
During the analysis of an unknown weak base B, a 0.10 m solution of the nitrate salt of the base was found to have a pH of 3.13. Use Table 6C.2 to identify the base.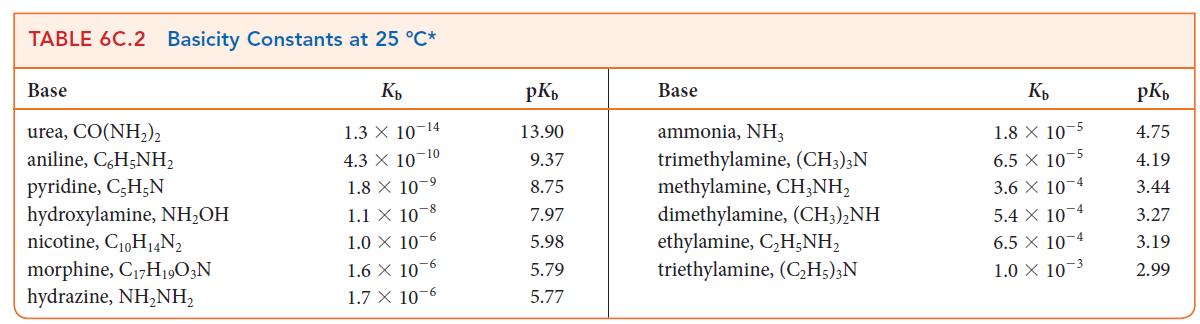
Transcribed Image Text:
TABLE 6C.2 Basicity Constants at 25 °C* Kb 1.3 X 10-14 4.3 X 10-10 1.8 X 10-9 1.1 X 10 8 1.0 x 10-6 1.6 x 10-6 1.7 X 10-6 Base urea, CO(NH,) aniline,C6H5NH₂ pyridine, C,H-N hydroxylamine, NH₂OH nicotine, C₁0H₁4N₂ morphine, C₁7H1903N hydrazine, NH₂NH₂ pKb 13.90 9.37 8.75 7.97 5.98 5.79 5.77 Base ammonia, NH3 trimethylamine, (CH3)3N methylamine, CH3NH₂ dimethylamine, (CH3)2NH ethylamine, C₂H₂NH₂ triethylamine, (C₂H5)3N Kb 1.8 X 10 5 6.5 X 105 3.6 X 10-4 5.4 x 10-4 6.5 x 10-4 1.0 X 10-3 pKb 4.75 4.19 3.44 3.27 3.19 2.99
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To identify the base B using the pH of its nitrate salt solution and Table 6C2 we need to understand the relationship between pH pOH and the pKb of th...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
During the analysis of an unknown acid HA, a 0.010 m solution of the sodium salt of the acid was found to have a pH of 10.35. Use Table 6C.1 to identify the acid. TABLE 6C.1 Acidity Constants at 25...
-
A student dissolves 0.0100 mol of an unknown weak base in 100.0 mL water and titrates the solution with 0.100 M HNO 3 . After 40.0 mL of 0.100 M HNO 3 was added, the pH of the resulting solution was...
-
Use Table 6H.2 to suggest suitable indicators for the titrations described in Exercises 6H.10 and 6H.12. Exercises 6H.10 Morphine, C 17 H 19 O 3 N, is a potent painkiller. Suppose you are studying...
-
What type of isomers are exhibited by [Fe(en) 3 ]Cl 2 (en = ethane-1,2-diamine)? no isomers are possible. cis and trans isomers fac and mer isomers optical isomers
-
In this problem you will derive an expression for the potential energy of a segment of a string carrying a traveling wave (Figure). The potential energy of a segment equals the work done by the...
-
Why was it profitable for General Motors and Ford to integrate backward into component-parts manufacturing in the past, and why are both companies now trying to buy more of their parts from outside?
-
Construct a bar graph for each of the following (assume the independent variable is group and the dependent variable is time): a. Group \(\mathrm{A}(N=5, M=4.00, s=1.58)\); Group B \((N=5, M=6.00,...
-
From the data 1,4, 5, 3, 2, 5, 7, 3, 4, and 5, Poindexter created the following frequency table. What five things did he do wrong? f- 013589 f- 11223|-| 123457
-
What single trade discount is equivalent to the series of trade discounts 14%, 9% and 5%? Enter your answer in the blank as a percent, rounded to 2 decimal places and including the percent sign.
-
Evie Excellent is trying to create financial statements for her start-up company, Stinky Soaps. Evie began the business on Jan 1, 2023 with a $1,000 investment from her parents. The year 2023 is...
-
The value of K w for water at body temperature (37C) is 2.1 * 10 14 . (a) What is the molar concentration of H 3 O + ions at 37C? (b) What is the molar concentration of OH in neutral water at 37 C?
-
Suggest an explanation for the different strengths of (a) Acetic acid and trichloroacetic acid; (b) Acetic acid and formic acid.
-
Most liquids follow Trouton's rule, which states that the molar entropy of vaporization lies in the range of 88 ± 5 J/mol-K. The normal boiling points and enthalpies of vaporization of several...
-
Swordfish are capable of stunning output power for short bursts. A 650 kg swordfish has a cross-section area of 0.92 m 2 and a drag coefficient of 0.0091exceptionally low due to a number of...
-
The Bod NATO base in northern Norway uses a heat pump to extract heat from ocean water. 7.0C ocean water is continuously drawn into the system, heat is extracted, and the water is returned to the...
-
The energy yield of a nuclear weapon is often defined in terms of the equivalent mass of a conventional explosive. 1 ton of a conventional explosive releases 4.2 GJ. A typical nuclear warhead...
-
Each time he does one pushup, Jose, who has a mass of 75 kg, raises his center of mass by 25 cm. He completes an impressive 150 pushups in 5 minutes, exercising at a steady rate. a. If we assume that...
-
A 10 kg migratory swan cruises at 20 m/s. A calculation that takes into account the necessary forces shows that this motion requires 200 W of mechanical power. If we assume an efficiency similar to...
-
Suppose you are testing H0: p = .29 versus Ha: p .29. A random sample of 740 items shows that 207 have this characteristic. With a .05 probability of committing a Type I error, test the hypothesis....
-
Why is a help desk and production support critical to system implementations? Discuss its interrelationship with the problem management and reporting system.
-
A glass bulb of volume 0.198 L contains 0.457 g of gas at 759.0 Torr and 134.0C. What is the molar mass of the gas?
-
Use LHpitals rule lim [f(x)/g(x)] x 0 = lim [df(x)dx/dg(x)/dx] x 0 to show that the expression derived for Pf in part b of Example problem 1.1 hane the correct limit as y 0.
-
For each compound below, identify all lone pairs and indicate whether each lone pair is localized or delocalized. Then, use that information to determine the hybridization state and geometry for each...
-
how can I recognize a preschool tearcher assistant in words for her amazing job performance?
-
Monello ( capital balance = $ 4 0 , 0 0 0 ) and Long ( capital balance = $ 5 0 , 0 0 0 ) and Kahn ( capital balance = $ 6 0 , 0 0 0 ) operate MLK Ventures. Long leaves the business and receives $ 6 ,...
-
4. Suppose X and Y are independent uniform random variables on [0, 1]. Let Z=XY. (a) Determine the cdf of Z. Suggestion: First compute P(Z > z) and pay attention to limits of integration (draw a...

Study smarter with the SolutionInn App


