Question: Balance the following equations and then write the net ionic equations. (Use the lowest possible whole number coefficients. Include states-of-matter in your answers. Use H+instead
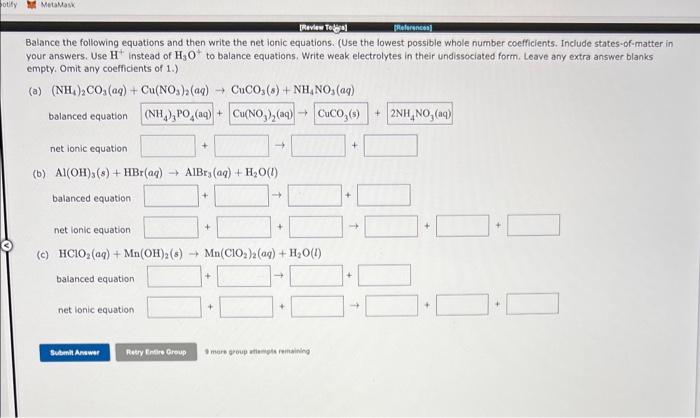
Balance the following equations and then write the net ionic equations. (Use the lowest possible whole number coefficients. Include states-of-matter in your answers. Use H+instead of H3O+to balance equations. Write weak electrolytes in their undissociated form. Leave any extra answer blanks empty. Omit any coefficients of 1. .) (a) (NH4)2CO3(aq)+Cu(NO3)2(aq)CuCO3(s)+NH4NO3(aq) balanced equation net ionic equation (b) Al(OH)3(s)+HBr(aq)AlBr3(aq)+H2O(l) balanced equation net lonic equation (c) HClO2(aq)+Mn(OH)2(s)Mn(ClO2)2(aq)+H2O(l) balanced equation net lonic equation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


