Question: Balance the following equations, using either the half-reaction method or the oxidation number method, and enter the coefficients in the boxes. The coefficients must
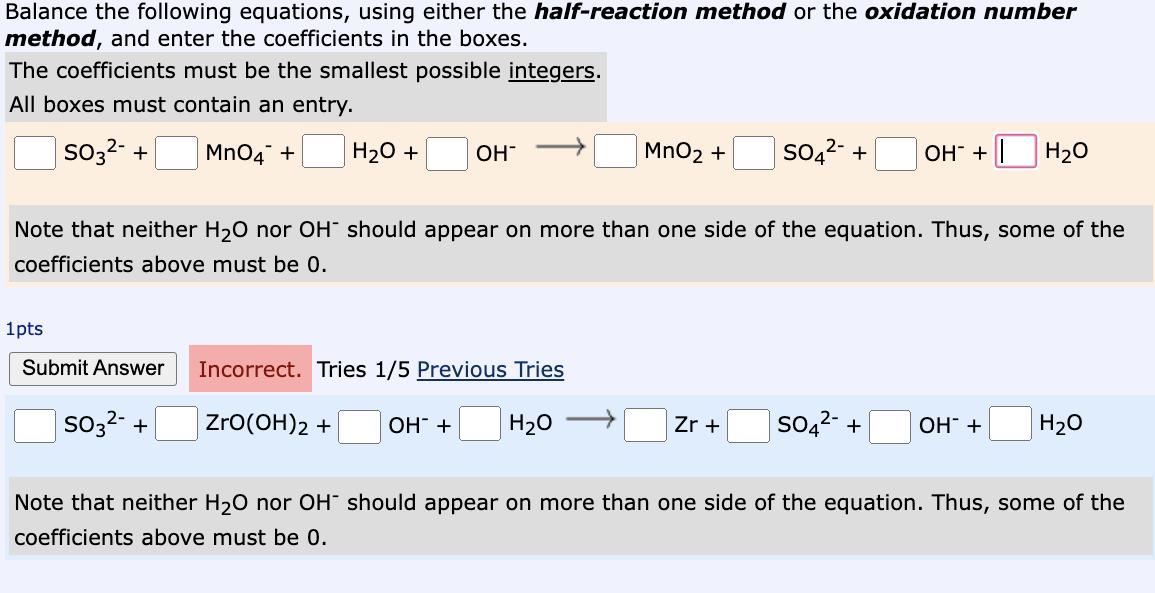
Balance the following equations, using either the half-reaction method or the oxidation number method, and enter the coefficients in the boxes. The coefficients must be the smallest possible integers. All boxes must contain an entry. SO3- + MnO4 + HO + OH Note that neither HO nor OH should appear on more than one side of the equation. Thus, some of the coefficients above must be 0. 1pts Submit Answer Incorrect. Tries 1/5 Previous Tries ZrO(OH)2 + HO SO3- + MnO + SO4- + OH- + | HO OH + Zr + SO4- + OH + HO Note that neither HO nor OH should appear on more than one side of the equation. Thus, some of the coefficients above must be 0.
Step by Step Solution
3.50 Rating (153 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


