Question: Based on the carefully performed experiments in a research laboratory, it was found that the nucleation rate of an active pharmaceutical ingredient 1 (API 1),
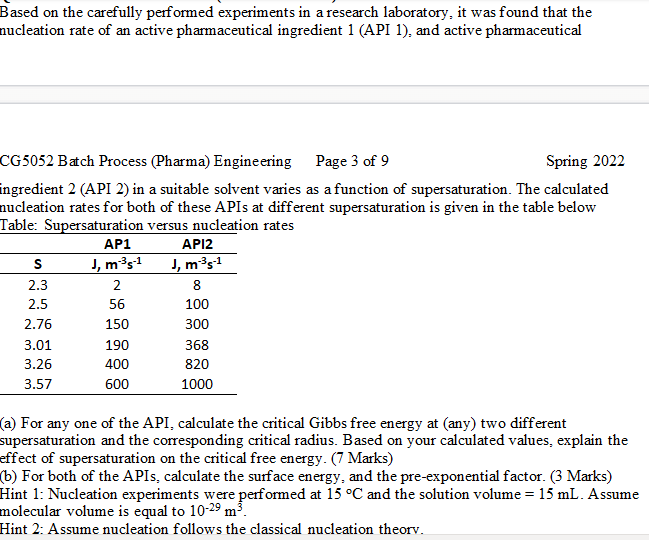
Based on the carefully performed experiments in a research laboratory, it was found that the nucleation rate of an active pharmaceutical ingredient 1 (API 1), and active pharmaceutical CG5052 Batch Process (Pharma) Engineering Page 3 of 9 Spring 2022 ingredient 2 (API 2 ) in a suitable solvent varies as a function of supersaturation. The calculated nucleation rates for both of these APIs at different supersaturation is given in the table below Table: Supersaturation versus nucleation rates (a) For any one of the API, calculate the critical Gibbs free energy at (any) two different supersaturation and the corresponding critical radius. Based on your calculated values, explain the effect of supersaturation on the critical free energy. (7 Marks) (b) For both of the APIs, calculate the surface energy, and the pre-exponential factor. ( 3 Marks) Hint 1: Nucleation experiments were performed at 15C and the solution volume =15mL. Assume molecular volume is equal to 1029m3. Hint 2: Assume nucleation follows the classical nucleation theorv
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


