Question: Based on the carefully performed experiments in a research laboratory, it was found that the nucleation rate of an active pharmaceutical ingredient 1 (API 1),
Based on the carefully performed experiments in a research laboratory, it was found that the nucleation rate of an active pharmaceutical ingredient 1 (API 1), and active pharmaceutical ingredient 2 (API 2) in a suitable solvent varies as a function of supersaturation. The calculated nucleation rates for both of these APIs at different supersaturation is given in the table below Table: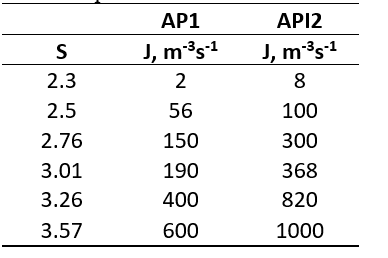
(a) For any one of the API, calculate the critical Gibbs free energy at (any) two different supersaturation and the corresponding critical radius.
\begin{tabular}{ccc} \hline & AP1 & API2 \\ \hline S & Jm3s1 & Jm3s1 \\ \hline 2.3 & 2 & 8 \\ 2.5 & 56 & 100 \\ 2.76 & 150 & 300 \\ 3.01 & 190 & 368 \\ 3.26 & 400 & 820 \\ 3.57 & 600 & 1000 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


