Question: Based on the following data, what is the Br-Br bond energy? 2 H2(g) + Br2(g) HBr(g); AH=-36.44 kJ/mol-rxn Bond Bond Energy (kJ/mol) - 435
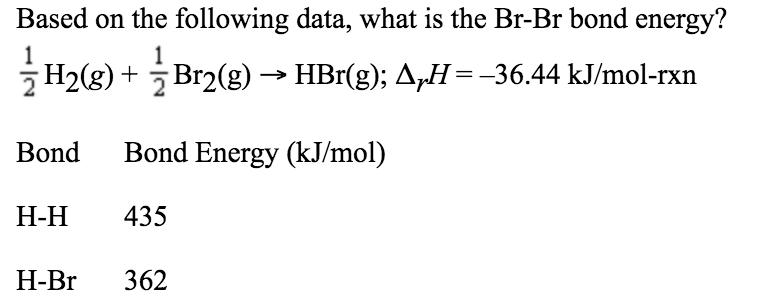
Based on the following data, what is the Br-Br bond energy? 2 H2(g) + Br2(g) HBr(g); AH=-36.44 kJ/mol-rxn Bond Bond Energy (kJ/mol) - 435 -Br 362
Step by Step Solution
There are 3 Steps involved in it
dH rxn BE of reactanats BE of pro... View full answer

Get step-by-step solutions from verified subject matter experts


