Question: Based on the phase diagram for methane below, what happens to methane as it is heated from absolute zero to room temperature at a pressure
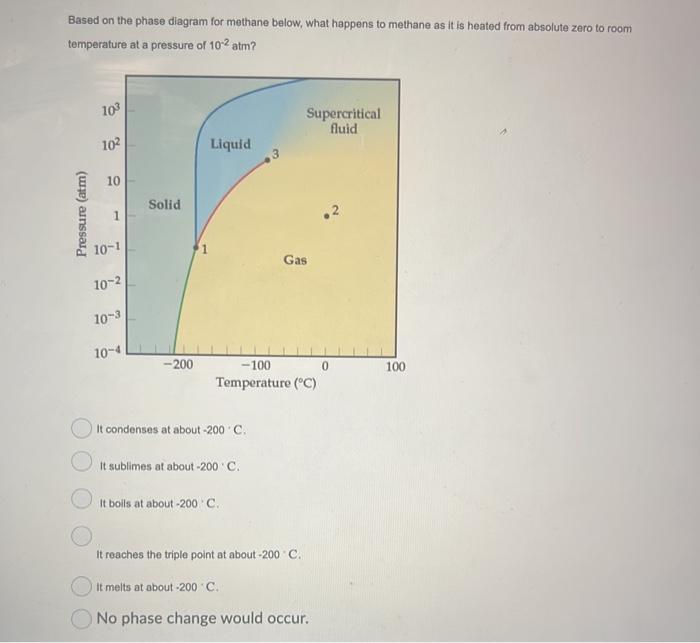
Based on the phase diagram for methane below, what happens to methane as it is heated from absolute zero to room temperature at a pressure of 102atm ? It condenses at about 200C. It sublimes at about 200. C. It boils at about 200 C. It reaches the triple point at about 200 - C. It melts at about 200C. No phase change would occur
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


