Question: Based on this weak acid titration curve, which statement is correct? more than one answer is correct (2 points) PPPPP b. pepe H 0-WA0V000-NU 0
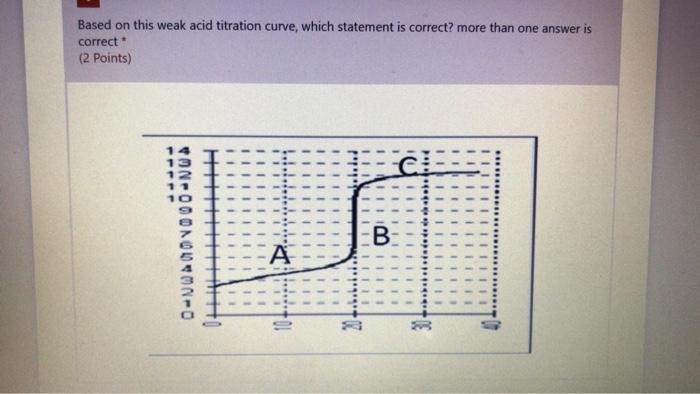
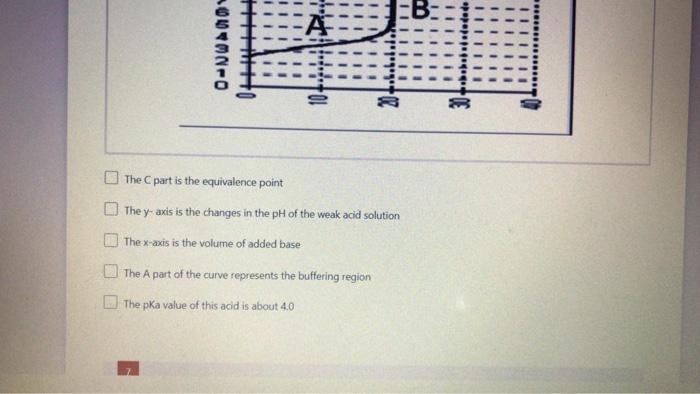
Based on this weak acid titration curve, which statement is correct? more than one answer is correct (2 points) PPPPP b. pepe H 0-WA0V000-NU 0 WI! B: A 04MNO The part is the equivalence point The y-axis is the changes in the pH of the weak acid solution The x-axis is the volume of added base The A part of the curve represents the buffering region The pKa value of this acid is about 4.0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


