Question: Be Bronz 1. To start this activity, elick this link for Counting Atoms (3) . The lab will load in a new tab. Click back
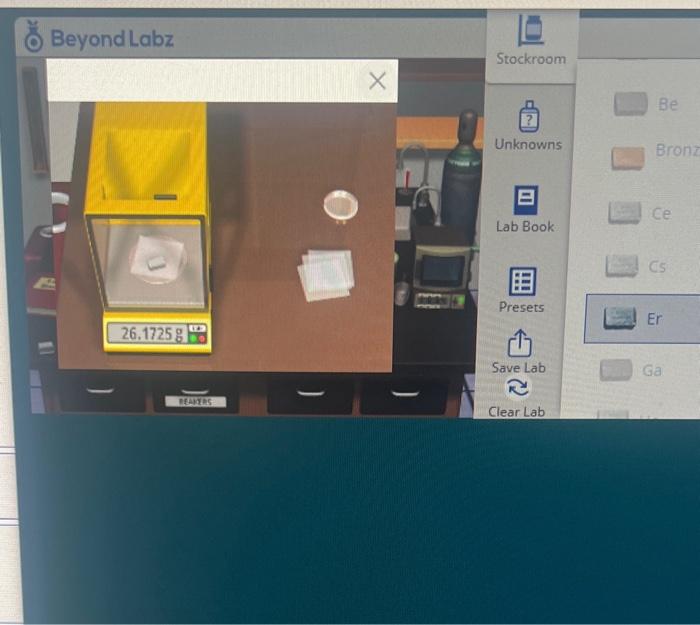
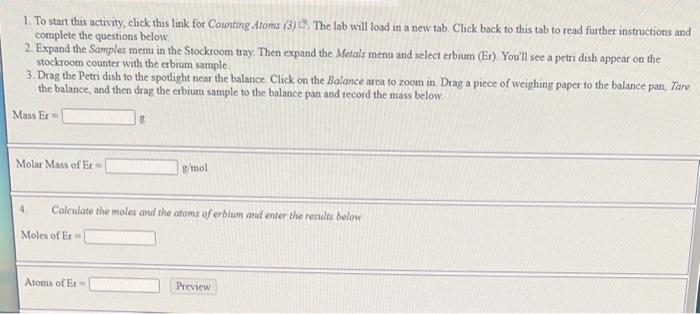
Be Bronz 1. To start this activity, elick this link for Counting Atoms (3) . The lab will load in a new tab. Click back to this tab to read further instructions and complete the questions below. 2. Expand the Samples menu in the Stockroom tray. Then expand the Metals menu and select erbium (Er). You'll see a petri dish appear on the stockroom counter with the erbium sample 3. Drag the Petri dish to the spotlight near the balance. Click on the Balance area to zoom in. Drag a piece of weighing paper to the balance pan, Tare the balance, and then drag the erbium sample to the balance pan and record the mass below Mass Er= Molar Mass of Er= g mol 4. Calculate the moles and the atoms of erbium and enter the results below Moles of Er=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


