Question: Be sure to answer all parts. (a) Write a balanced equation for the reaction depicted below. Do not include states. (b) If each reactant molecule
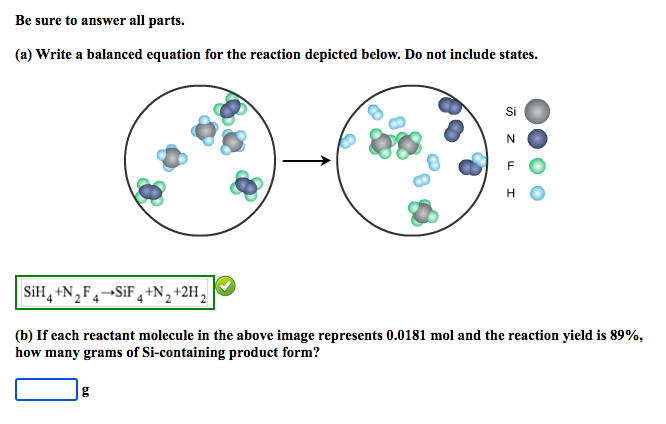
Be sure to answer all parts. (a) Write a balanced equation for the reaction depicted below. Do not include states. (b) If each reactant molecule in the above image represents 0.0181mol and the reaction yield is 89%, how many grams of Si-containing product form
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


