Question: Beaker for top section Beaker for bottom section Standardized NaOH(M) Initial volume of buret (mL) Volume of vinegar (mL) Observations Final volume of buret (mL)
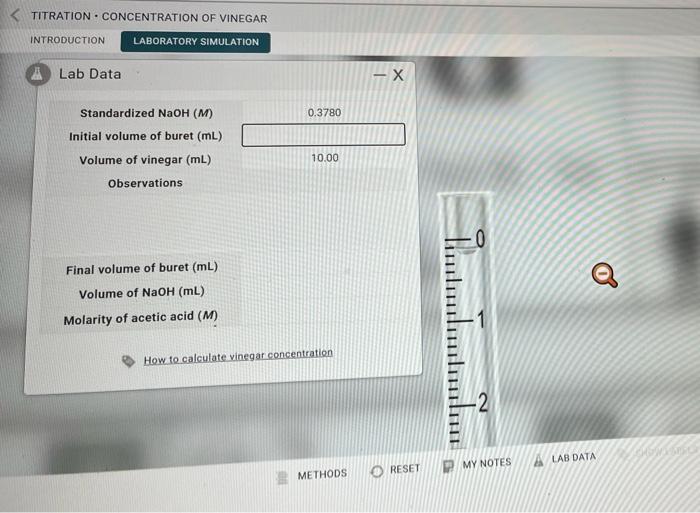
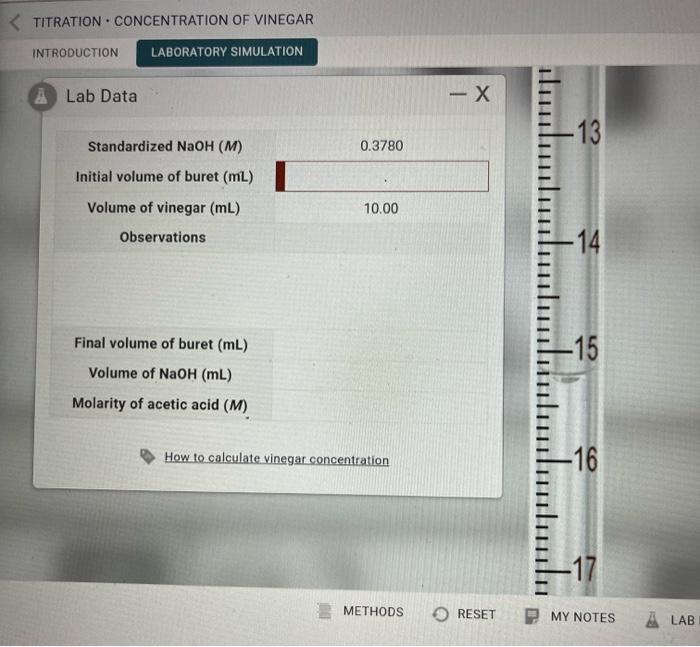
Standardized NaOH(M) Initial volume of buret (mL) Volume of vinegar (mL) Observations Final volume of buret (mL) Volume of NaOH(mL) Molarity of acetic acid (M) TITRATION CONCENTRATION OF VINEGAR INTRODUCTION LABORATORY SIMULATION Standardized NaOH(M) Initial volume of buret (mL) Volume of vinegar (mL) Observations Final volume of buret (mL) Volume of NaOH(mL) Molarity of acetic acid (M) How to calculate vinegar concentration
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


