Question: [ begin{array}{l} text { Point } mathrm{A}=ldots text { Furfural,___ glycol,___ Water } text { Point } mathrm{B}=ldots text { Furfural,___ } % text
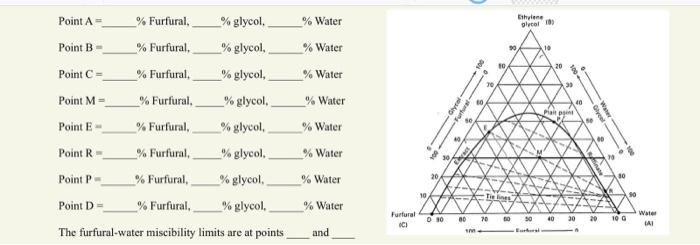
\[ \begin{array}{l} \text { Point } \mathrm{A}=\ldots \text { Furfural,___ glycol,___ Water } \\ \text { Point } \mathrm{B}=\ldots \text { Furfural,___ } \% \text { glycol, ___ Water } \\ \text { Point } \mathrm{C}=\ldots \text { Furfural, } \_\% \text { glycol, ___ } \% \text { Water } \\ \text { Point } \mathrm{M}=\ldots \text { __ Furfural,___ } \% \text { glycol,___ } \% \text { Water } \\ \text { Point } \mathrm{E}=\ldots \text { \%urfural,__ \% glycol, ___ } \% \text { Water } \\ \text { Point } \mathrm{R}=\_\% \text { Furfural,___ glycol, } \% \_\% \text { Water } \\ \text { Point } \mathrm{P}=\ldots \text { Furfural,___ glycol,___ } \% \text { Water } \\ \text { Point } \mathrm{D}=\ldots \text { Furfural, } \_\% \text { glycol,___ } \% \text { Water } \\ \end{array} \] The furfural-water miscibility limits are at points and
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


