Question: Below are two resonance structures for formic acid. Part A oxygen atom is more electronegative than the C and H hydrogen atom is more
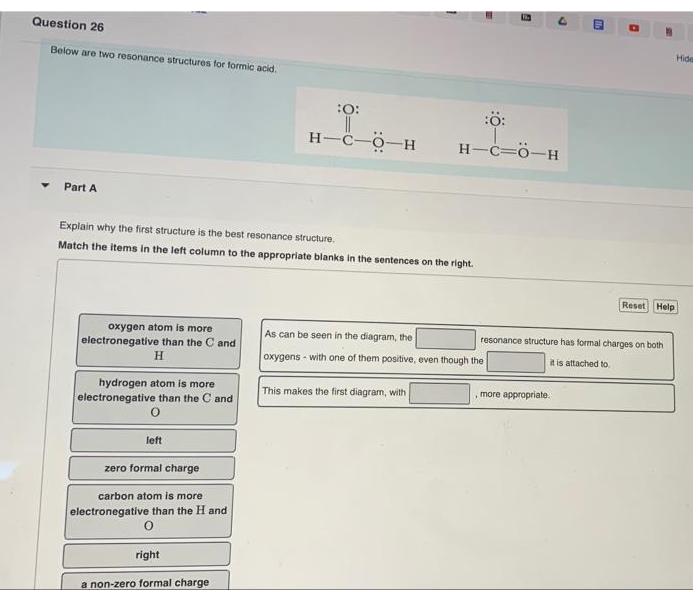
Below are two resonance structures for formic acid. Part A oxygen atom is more electronegative than the C and H hydrogen atom is more electronegative than the C and Explain why the first structure is the best resonance structure. Match the items in the left column to the appropriate blanks in the sentences on the right. left zero formal charge carbon atom is more electronegative than the H and right :O: a non-zero formal charge H-C-O-H m As can be seen in the diagram, the oxygens- with one of them positive, even though the This makes the first diagram, with 6 :: H-C=O-H 4 , more appropriate. Reset Help resonance structure has formal charges on both it is attached to Hide
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
The detailed ... View full answer

Get step-by-step solutions from verified subject matter experts


