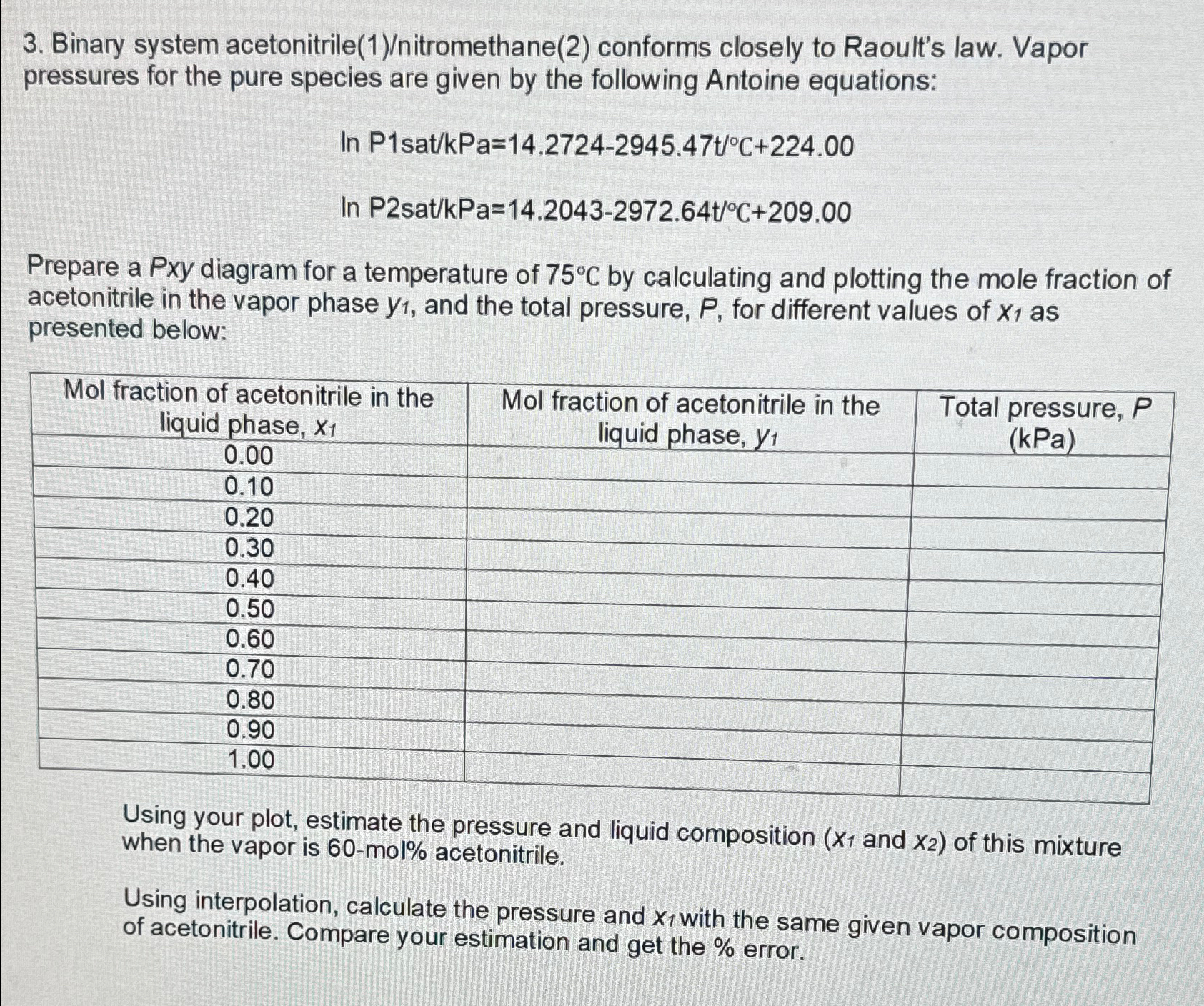
Question: Binary system acetonitrile ( 1 ) / nitromethane ( 2 ) conforms closely to Raoult's law. Vapor pressures for the pure species are given by
Binary system acetonitrilenitromethane conforms closely to Raoult's law. Vapor pressures for the pure species are given by the following Antoine equations:
Prepare a Pxy diagram for a temperature of by calculating and plotting the mole fraction of acetonitrile in the vapor phase and the total pressure, for different values of as presented below:
tabletableMol fraction of acetonitrile in theliquid phase,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


