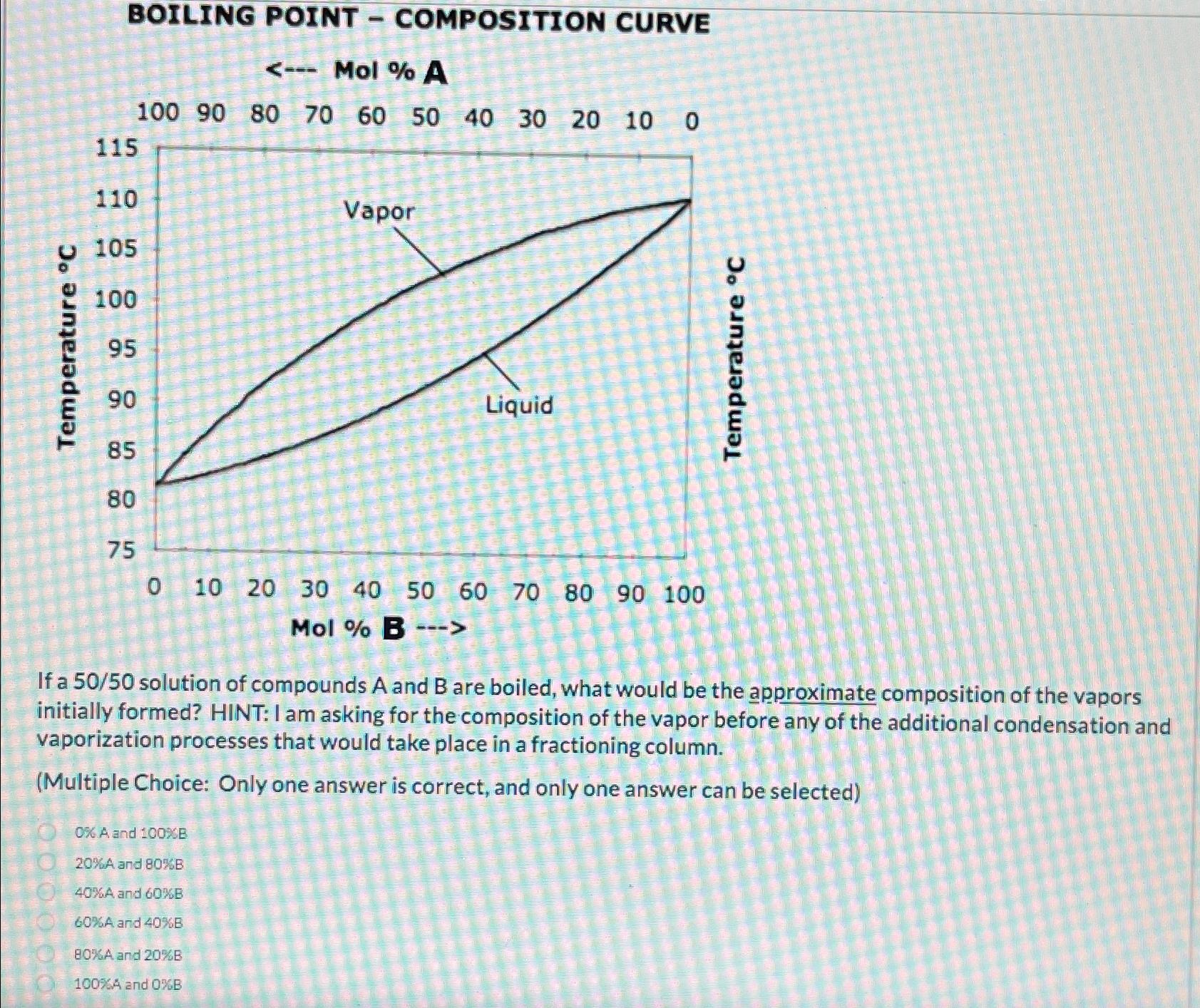
Question: BOILING POINT - COMPOSITION CURVE Mol % A If a 5 0 / 5 0 solution of compounds A and B are boiled, what would
BOILING POINT COMPOSITION CURVE
Mol A
If a solution of compounds A and are boiled, what would be the approximate composition of the vapors initially formed? HINT: I am asking for the composition of the vapor before any of the additional condensation and vaporization processes that would take place in a fractioning column.
Multiple Choice: Only one answer is correct, and only one answer can be selected
OA and
A and
A and
A and
A and B
A and
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


