Question: Boiling Point Elevation The boiling point of a solution INCREASES with the number of dissolved solute particles. This is summarized by the expression: Tb=m i


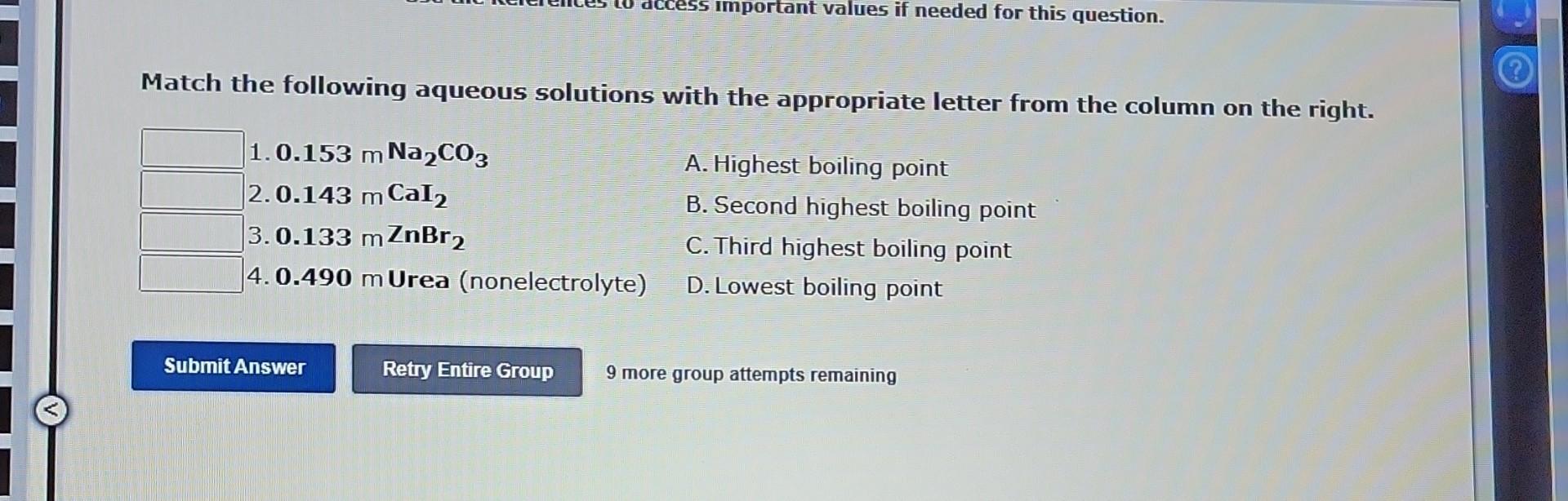
Boiling Point Elevation The boiling point of a solution INCREASES with the number of dissolved solute particles. This is summarized by the expression: Tb=m i Kb Kb is the boiling point elevation constant. It depends only on the SOLVENT. Example: Na2SO4 is a soluble salt. It breaks into two Na+cations and one SO42 anion in solution. i=2+1=3 CH3COOH is a weak acid. It breaks into a few CH3COO - anions and a few H+cations. 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


