Question: Cha11 Q1 Match them please When a solution contains the maximum concentration of solute possible for a given temperature and pressure the ratio of the

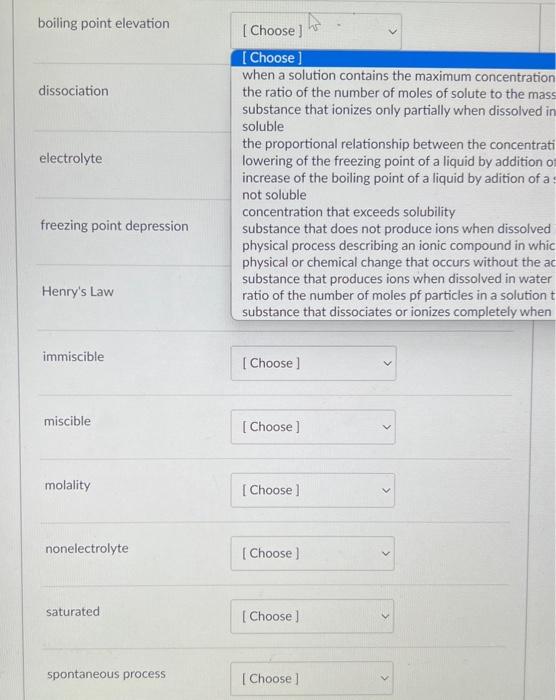
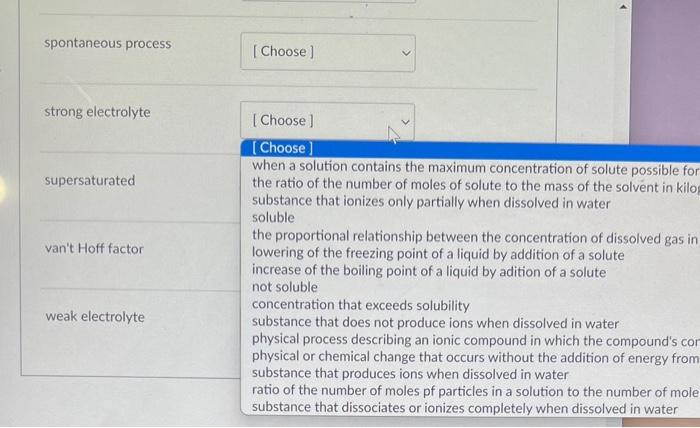
When a solution contains the maximum concentration of solute possible for a given temperature and pressure the ratio of the number of moles of solute to the mass of the solvent in kilograms substance that ionizes only partially when dissolved in water soluble the proportional relationship between the concentration of dissolved gas in a solution and the partial pressure of the gas in contact with the solutio lowering of the freezing point of a liquid by addition of a solute increase of the boiling point of a liquid by adition of a solute not soluble concentration that exceeds solubility substance that does not produce ions when dissolved in water physical process describing an ionic compound in which the compound's constituent ions are solvated and dispersed throughout the solution physical or chemical change that occurs without the addition of energy from an external source substance that produces ions when dissolved in water ratio of the number of moles pf particles in a solution to the number of moles of formula units dissolved in the solution substance that dissociates or ionizes completely when dissolved in water boiling point elevation [Choose] when a solution contains the maximum concentration the ratio of the number of moles of solute to the mas: substance that ionizes only partially when dissolved ir soluble the proportional relationship between the concentrat lowering of the freezing point of a liquid by addition o increase of the boiling point of a liquid by adition of a not soluble concentration that exceeds solubility substance that does not produce ions when dissolved physical process describing an ionic compound in whic physical or chemical change that occurs without the at substance that produces ions when dissolved in water ratio of the number of moles pf particles in a solution substance that dissociates or ionizes completely when immiscible miscible molality nonelectrolyte saturated spontaneous process spontaneous process strong electrolyte [Choose] when a solution contains the maximum concentration of solute possible for the ratio of the number of moles of solute to the mass of the solvent in kilo substance that ionizes only partially when dissolved in water soluble the proportional relationship between the concentration of dissolved gas in lowering of the freezing point of a liquid by addition of a solute increase of the boiling point of a liquid by adition of a solute not soluble concentration that exceeds solubility substance that does not produce ions when dissolved in water physical process describing an ionic compound in which the compound's cor physical or chemical change that occurs without the addition of energy from substance that produces ions when dissolved in water ratio of the number of moles pf particles in a solution to the number of mole substance that dissociates or ionizes completely when dissolved in water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


