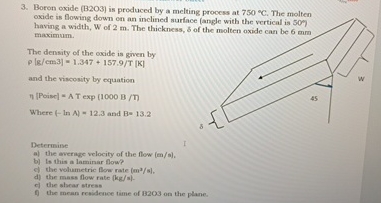
Question: Boron oxide ( B 2 O 3 ) is produced by a melting process at 7 5 0 C . The molten oxide is flowing
Boron oxide BO is produced by a melting process at The molten oxide is flowing down on an inclined surface angle with the vertical is deg having a width, W of m The thickness, the molten oxide can be mm maximum.
The density of the oxcide is given by
and the viscosity by equation
Where and
Determine
a the average velocity of the flow
b It ahis a Inminar fow?
d the volumetric Aow rate
d the mass flow rate
e the shear stress
he mean residence time of BO on the plane.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


