Question: Buffer Solutions Buffer solutions: pH after addition of 3.0 MHICI Beaker Initial pil after addition of pH after # pH 5 drops HCI 10 drops
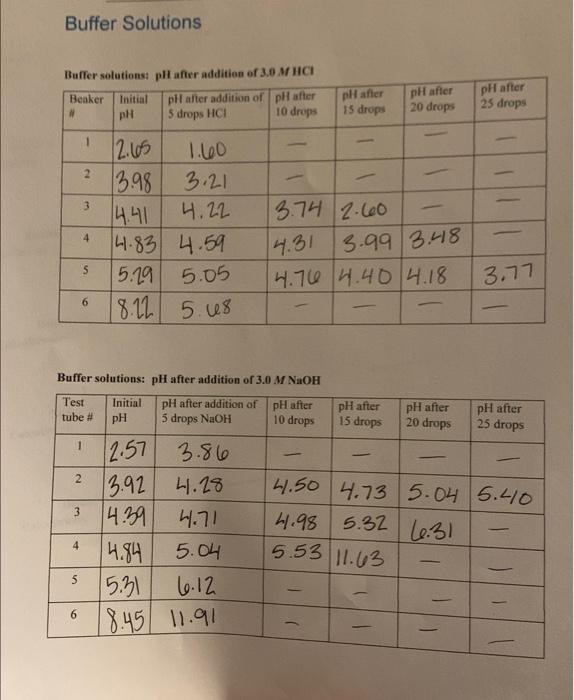



Buffer Solutions Buffer solutions: pH after addition of 3.0 MHICI Beaker Initial pil after addition of pH after # pH 5 drops HCI 10 drops pH after pH after pH after 15 drops 20 drops 25 drops 1 2 3 3.74 2.60 2.65 1.60 13.98 3.21 4.41 4.22 4.83 4.59 5.19 5.05 18.22 5.us 4 14.31 3.99 3.48 4.76 4.404.18 5 3.77 6 Buffer solutions: pH after addition of 3.0 M NaOH Test Initial pH after addition of pH after tube # pH 5 drops NaOH pH after pH after pH after 10 drops 15 drops 20 drops 25 drops 1 2 3 12.57 3.86 13.92 4.28 14:39 4.71 4.84 5.04 15.31 6.12 18.45 11.91 14.50 4.73 5.04 5.40 4.98 5.32 16.31 4 5.53 11.63 5 6 - - Concentrations of HC,H,O, and CH.0.) in each beaker: THCHO, ICHO, 7 Beaker #1 Calculations: Beaker #2 Calculations: Beaker #3 Calculations: Beaker #4 Calculations: Beaker #5 Calculations: Beaker #6 Calculations: Report Sheet for E Comparison of experimental and theoretical pH's Measured pH Calculated pH ApH Beaker #1 Calculations: Beaker #2 Calculations: 1 Beaker #3 Calculations: Beaker #4 Calculations: Beaker #5 Calculations: Beaker #6 Calculations: Report Sheet for EXP5 Buffer capacities for each solution: Acid buffer capacity Base buffer capacity Beaker # 1 Beaker #2 Beaker #3 Beaker #4 Beaker #5 Beaker #6 Observations about any patterns between buffer capacities and the concentration of acid or base in each buffer solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


