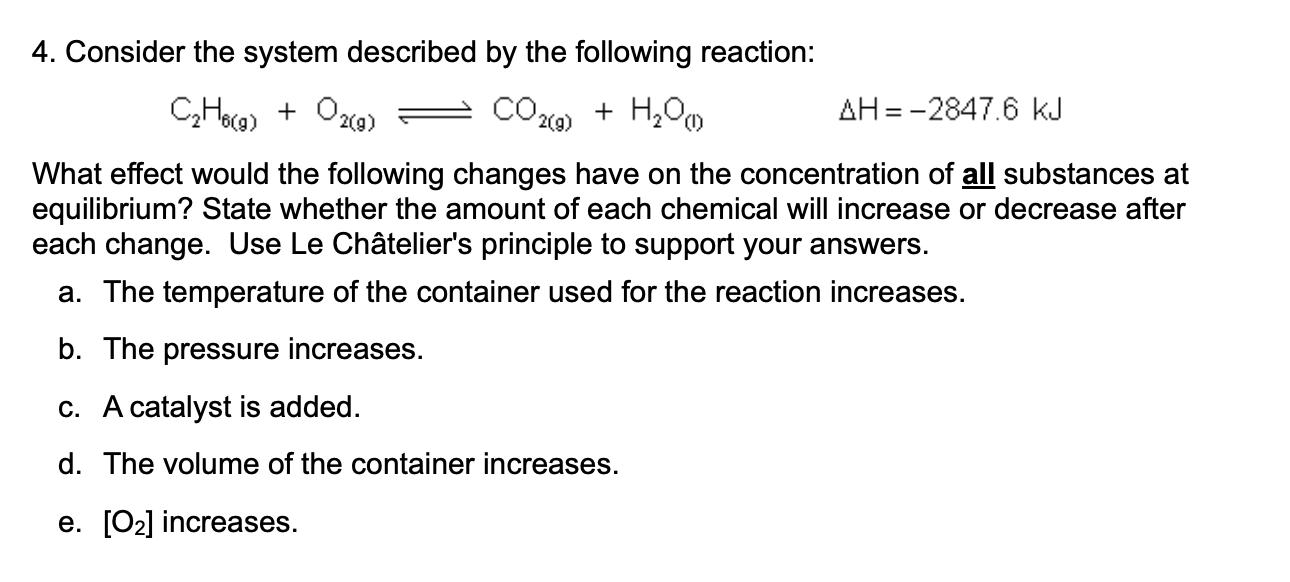
Question: C 2 H 6 ( g ) + O 2 ( g ) C O 2 ( g ) + H 2 O ( l
What effect would the following changes have on the concentration of all substances at
equilibrium? State whether the amount of each chemical will increase or decrease after
each change. Use Le Chteliers principle to support your answers.
a The temperature of the container used for the reaction increases.
b The pressure increases.
c A catalyst is added.
d The volume of the container increases.
e increases.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


