Question: ( c ) Consider the isothermal gas phase reaction A + 3 B 6 C ; and the feed consists of C A = 1
c Consider the isothermal gas phase reaction ; and the feed consists of liter, moleliter molefliter to a steady state CSTR operated at constant pressure. If is litre, determine the following: iiiiii Volume of CSTR
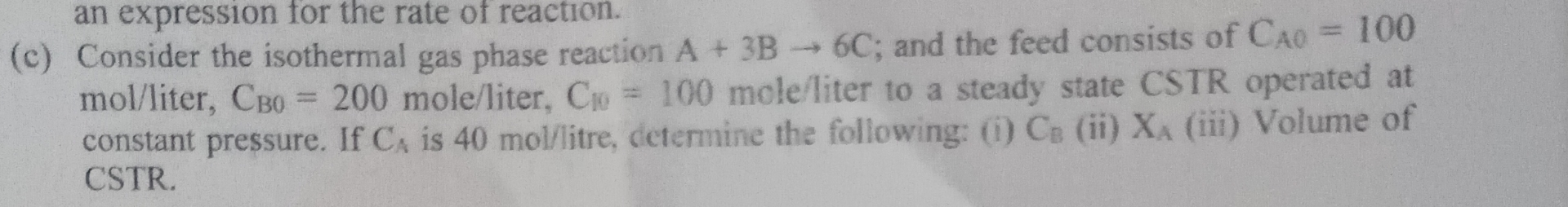
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


