Question: (c) Example 6-3. Download the Living Example Problem 6-3 from the CRE Web site. The temperature is to be lowered by 35C so that the

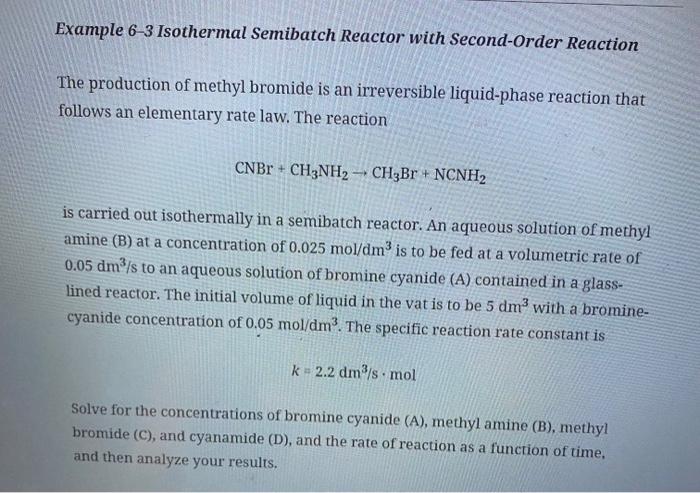
(c) Example 6-3. Download the Living Example Problem 6-3 from the CRE Web site. The temperature is to be lowered by 35C so that the reaction-rate constant is now one-tenth its original value. (1) If the concentration of B is to be maintained at 0.01mol/dm3 or below, what is the maximum feed rate of B?(2) How would your answer change if the concentration of A were tripled? (2 : Example 6-3 Isothermal Semibatch Reactor with Second-Order Reaction The production of methyl bromide is an irreversible liquid-phase reaction that follows an elementary rate law. The reaction CNBr+CH3NH2CH3Br+NCNH2 is carried out isothermally in a semibatch reactor. An aqueous solution of methyl amine (B) at a concentration of 0.025mol/dm3 is to be fed at a volumetric rate of 0.05dm3/s to an aqueous solution of bromine cyanide (A) contained in a glasslined reactor. The initial volume of liquid in the vat is to be 5dm3 with a brominecyanide concentration of 0.05mol/dm3. The specific reaction rate constant is k=2.2dm/smol Solve for the concentrations of bromine cyanide (A), methyl amine (B), methyl bromide (C), and cyanamide (D), and the rate of reaction as a function of time, and then analyze your results
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


