Question: C https://learn hawkeslearning.com/Portal/Lesson/lesson_certify 3/15 Question 4 of 15, Step 1 of 1 Correct In chemistry, the pH of a solution is a measure of the
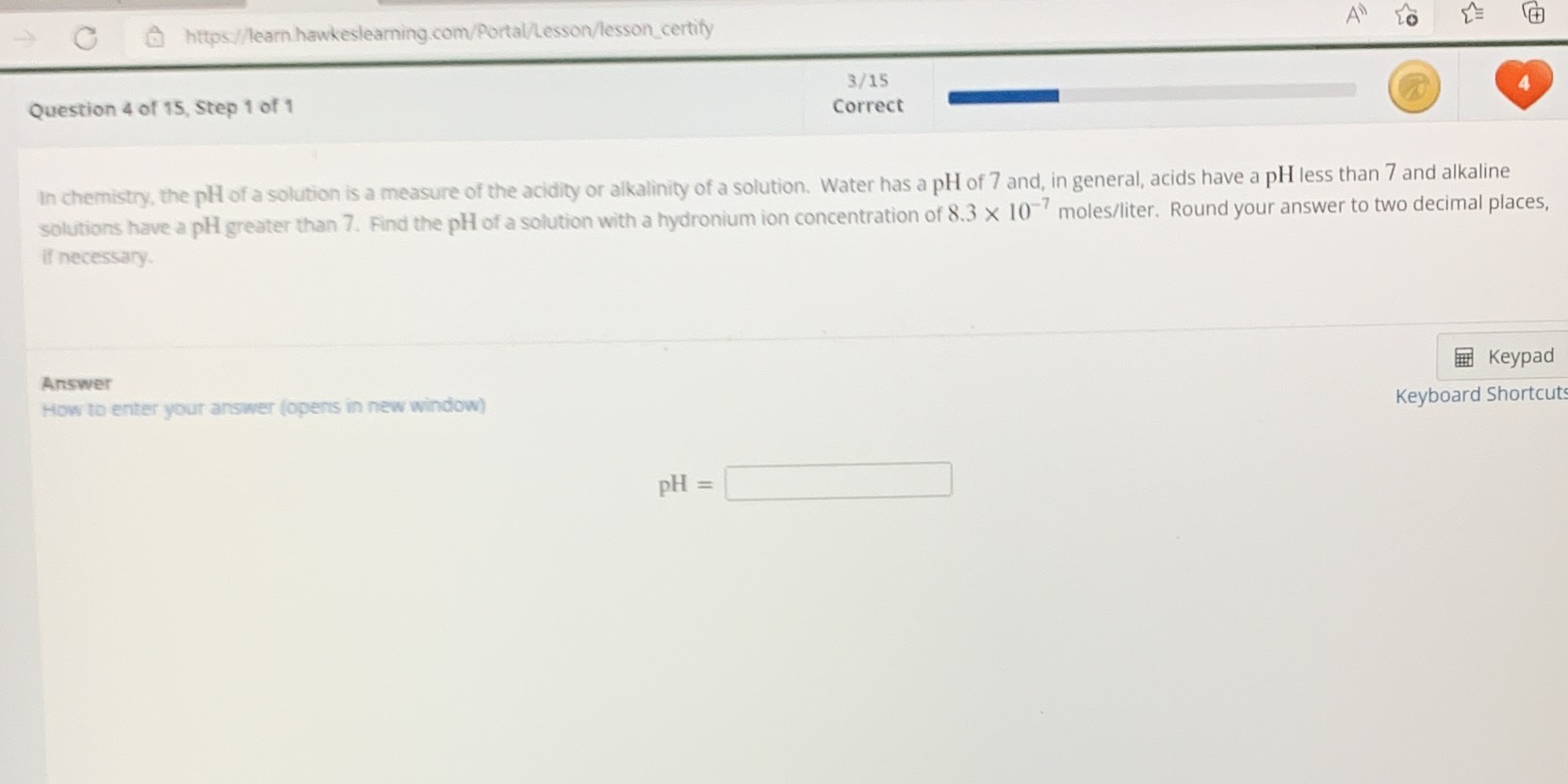
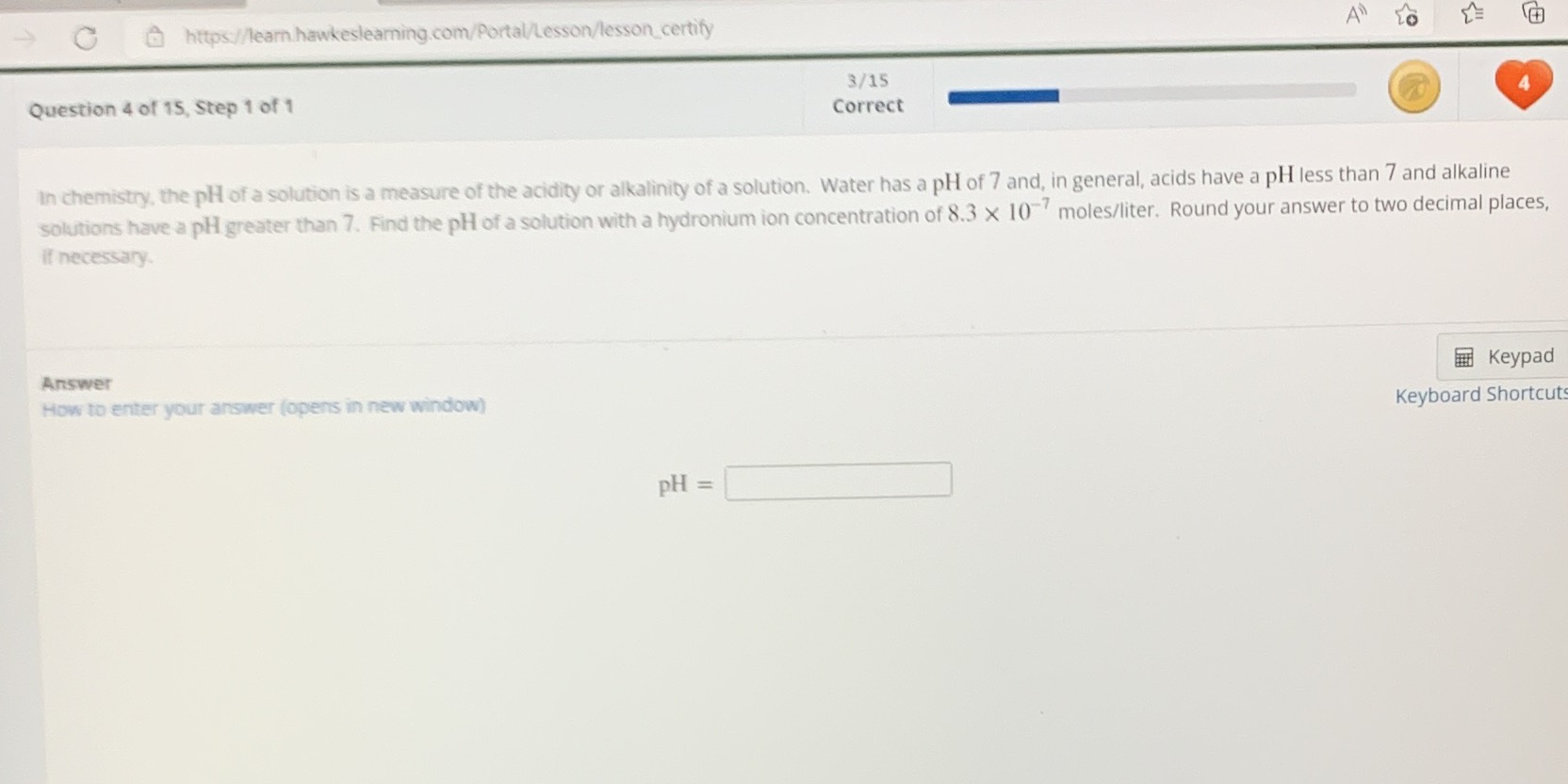
C https://learn hawkeslearning.com/Portal/Lesson/lesson_certify 3/15 Question 4 of 15, Step 1 of 1 Correct In chemistry, the pH of a solution is a measure of the acidity or alkalinity of a solution. Water has a pH of 7 and, in general, acids have a pH less than 7 and alkaline solutions have a pl greater than 7. Find the pH of a solution with a hydronium ion concentration of 8.3 X 10 moles/liter. Round your answer to two decimal places, if necessary. Answer Keypad How to enter your answer (opens in new window) Keyboard Shortcut PH =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


