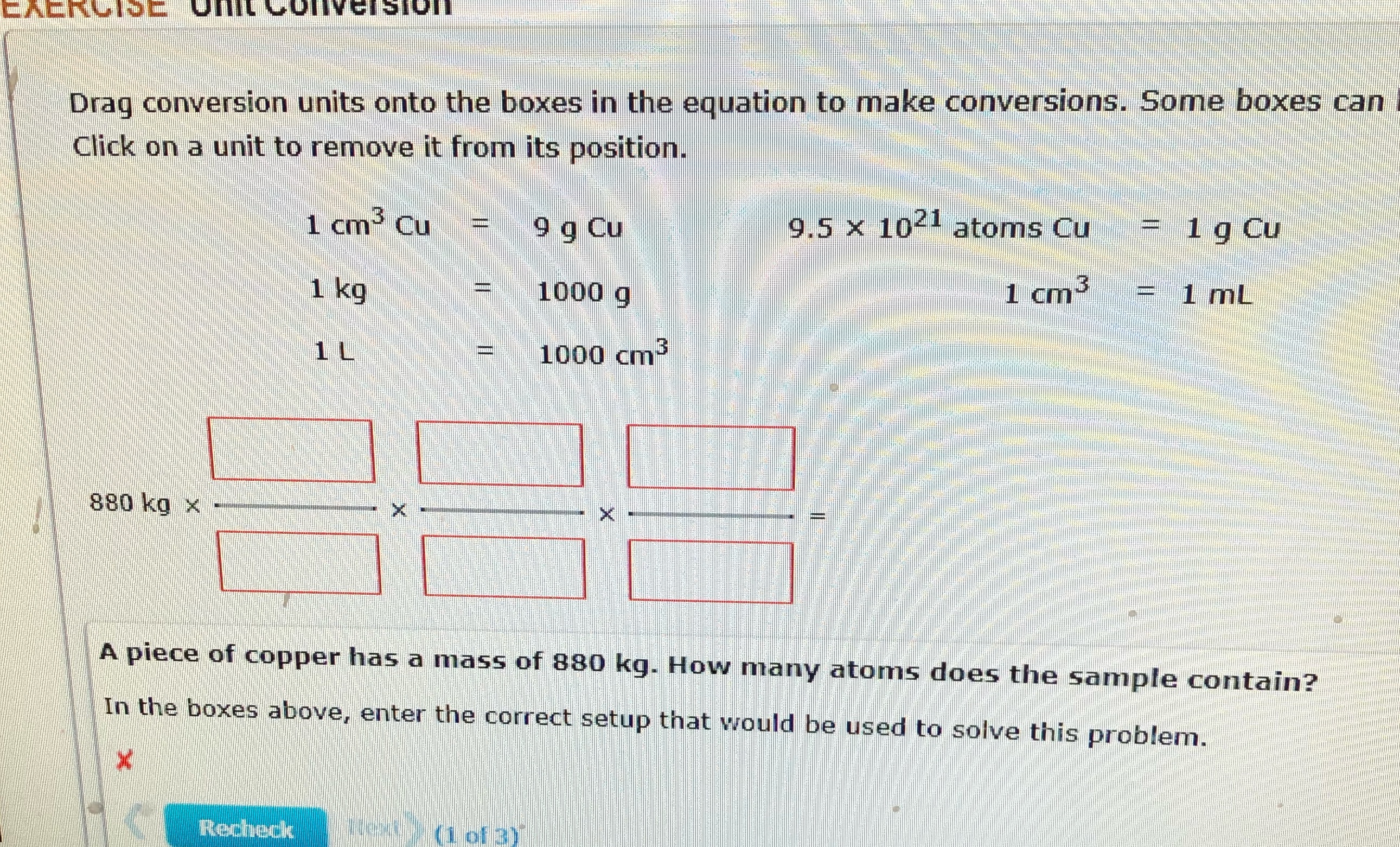
Question: CACKCISE Of CONVERSION Drag conversion units onto the boxes in the equation to make conversions. Some boxes can Click on a unit to remove it
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


