Question: Calculate the bubble point temperature (TBP, bubble point temp.) at 101.3 kPa of the liquid mixture containing 30% acetone, 30% acetonitrile and 40% nitromethane as
Calculate the bubble point temperature (TBP, bubble point temp.) at 101.3 kPa of the liquid mixture containing 30% acetone, 30% acetonitrile and 40% nitromethane as mole percent and the composition of the vapor phase formed. If the mixture were a vapor mixture containing 30% acetone, 30% acetonitrile and 40% nitromethane in mole percent, what would be the condensation temperature (TDP, dew point temp.) at 101.3 kPa and the composition of the resulting liquid phase?
a) Calculate analytically.
b) Calculate using a computer program.
c) Draw graphs of T versus xacetone, xacetonitrile, xnitromethane, and T versus yacetone, yacetonitrile, nitromethane.
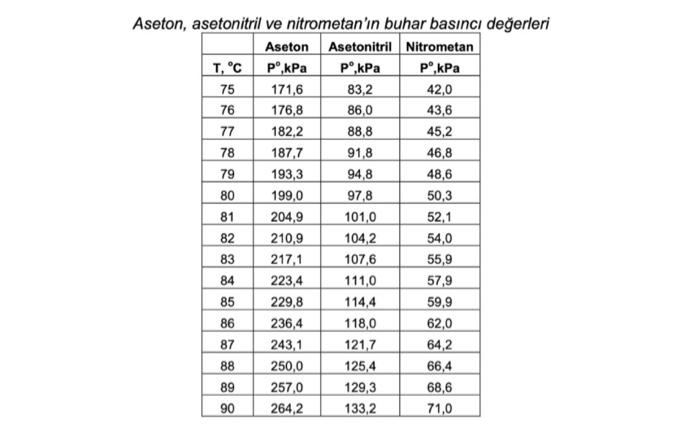
Aseton, asptonitril ve nitmmetan'in huhar hasnc deerleri
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


