Question: Calculate the enthalpy change, H, for the process in which 11.8g of water is converted from liquid at 20.0C to vapor at 25.0C. For water,
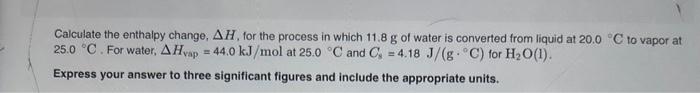
Calculate the enthalpy change, H, for the process in which 11.8g of water is converted from liquid at 20.0C to vapor at 25.0C. For water, Hvap=44.0kJ/mol at 25.0C and Cs=4.18J/(gC) for H2O(1). Express your answer to three significant figures and include the appropriate units
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


